Effect of Nd-incorporation and K-modification on catalytic performance of Co3O4 for N2O decomposition
-
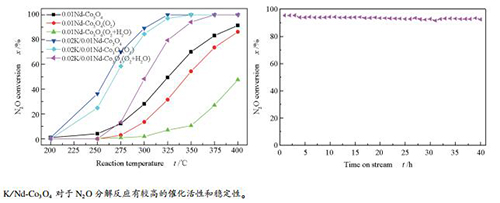
摘要: 用水热法和共沉淀法分别制备了Nd-Co3O4催化剂,催化分解N2O。其中,水热法制备的Nd-Co3O4催化活性较高。在不同组成的Nd-Co3O4中,优化出了较高活性的0.01Nd-Co3O4催化剂,在其表面浸渍K2CO3溶液制备K改性催化剂(K/Nd-Co3O4)。用X射线衍射(XRD)、N2物理吸附、扫描电镜(SEM)、X射线光电子谱(XPS)、程序升温还原(H2-TPR)、O2程序升温脱附(O2-TPD)等技术表征催化剂结构。结果表明,Nd-Co3O4和K改性催化剂均为尖晶石结构;K改性弱化了催化剂表面Co-O键,有利于表面氧的脱除,提高了催化剂活性。有氧有水气氛350 ℃连续反应40 h,K/Nd-Co3O4催化剂上的N2O分解率超过90%,稳定性较好。Abstract: Nd-Co3O4 catalysts were prepared by hydrothermal and co-precipitation methods to catalyze the decomposition of N2O. The catalysts prepared by hydrothermal method showed higher activity. Among the hydrothermal Nd-Co3O4 catalysts, the catalyst with Nd/Co molar ratio of 0.01 had higher activity. 0.01Nd-Co3O4 catalyst was then impregnated by K2CO3 solution to prepare K-modified catalyst. The catalysts were characterized by means of X-ray diffraction (XRD), nitrogen physisorption, scanning electrons microscopy (SEM), X-ray photoelectron spectroscopy (XPS), hydrogen temperature-programmed reduction (H2-TPR), and oxygen temperature-programmed desorption (O2-TPD). The results show that Nd-Co3O4 and K-modified catalysts exhibit spinel structure. In contrast to bare Nd-Co3O4, the K-modified catalyst with higher activity is due to its weaker strength of Co-O bond and easier desorption of surface oxygen species. In addition, over 90% conversion of N2O can be reached over 0.02K/0.01Nd-Co3O4 at 350 ℃ for 40 h under the co-presence of oxygen and steam in feed gases.
-
Table 1 Crystallite size and specific surface area of Nd-Co3O4 with various compositions
Catalyst Crystallite size d/nm Specific surface area A/(m2·g-1) Nd/Co=0 (Co3O4) 26.8 28.5 Nd/Co=0.01 19.7 48.0 Nd/Co=0.03 23.4 50.7 Nd/Co=0.05 24.7 47.4 Table 2 Kinetic data of N2O decomposition on Nd-Co3O4 catalysts with various compositions
Catalyst k/s-1 Ea/(kJ·mol-1) lnA 300 ℃ 325 ℃ 350 ℃ 375 ℃ Nd/Co=0 (Co3O4) 0.67 1.37 3.02 4.73 82.4 16.9 Nd/Co=0.01 1.90 3.59 5.97 8.78 63.3 14.0 Nd/Co=0.03 1.23 2.80 4.95 7.83 76.0 16.2 Nd/Co=0.05 1.22 2.65 4.68 7.19 73.0 15.6 Table 3 XPS data of Nd-Co3O4 catalysts with various compositions
Catalyst Binding energies of Co 2p3/2 E /eV Co2+/Co3+ (molar ratio) Co2+ Co3+ Nd/Co=0 (Co3O4) 779.78 781.39 1.48 Nd/Co=0.01 779.72 781.32 1.59 Nd/Co=0.03 779.65 781.26 1.58 Nd/Co=0.05 779.67 781.21 1.45 Table 4 XPS data of Nd-Co3O4 and K-modified catalysts
Catalyst Binding energies of Co 2p3/2 E /eV Co2+/Co3+ (molar ratio) Co2+ Co3+ 0.01Nd-Co3O4 779.72 781.32 1.59 0.02K/0.01Nd-Co3O4 779.63 781.22 1.59 -
[1] REILLY J, PRINN R, HARNISCH J, FITZMAURICE J, JACOBY H, KICKLIGHTER D, MELILLO J, STONE P, SOKOLOV A, WANG C. Multi-gas assessment of the Kyoto Protocol[J]. Nature, 1999, 401:549-555. doi: 10.1038/44069 [2] SHEN Q, LI L D, LI J J, TIAN H, HAO Z P. A study on N2O catalytic decomposition over Co/MgO catalysts[J]. J Hazard Mater, 2009, 163:1332-1337. doi: 10.1016/j.jhazmat.2008.07.104 [3] YAN L, REN T, WANG X L, JI D, SUO J S. Catalytic decomposition of N2O over MxCo1-xCo2O4(M=Ni, Mg) spinel oxides[J]. Appl Catal B:Environ, 2003, 45(2):85-90. doi: 10.1016/S0926-3373(03)00174-7 [4] IVANOVA Y A, SUTORMINA E F, ISUPOVA L A, ROGOV V A. Effect of the composition of NixCo3-xO4(x=0-0.9) oxides on their catalytic activity in the low-temperature reaction of N2O decomposition[J]. Kinet Catal, 2018, 59(3):365-370. doi: 10.1134%2FS0023158418030072 [5] DOU Z, ZHANG H J, PAN Y F, XU X F. Catalytic decomposition of N2O over potassium-modified Cu-Co spinel oxides[J]. J Fuel Chem Technol, 2014, 42(2):238-245. doi: 10.1016/S1872-5813(14)60016-5 [6] FRANKEN T, PALKOVITS R. Investigation of potassium doped mixed spinels CuxCo3-x O4 as catalysts for an efficient N2O decomposition in real reaction conditions[J]. Appl Catal B:Environ, 2015, 176/177:298-305. doi: 10.1016/j.apcatb.2015.04.002 [7] WANG Y Z, HUO X B, ZHANG K, WEI X H, ZHAO Y X. Effect of SnO2 on the structure and catalytic performance of Co3O4 for N2O decomposition[J]. Catal Commun, 2018, 111:70-74. doi: 10.1016/j.catcom.2018.04.004 [8] TURSUN M, WANG X P, ZHANG F, YU H B. Bi-Co3O4 catalyzing N2O decomposition with strong resistance to CO2[J]. Catal Commun, 2015, 65:1-5. doi: 10.1016/j.catcom.2015.02.013 [9] YU H B, TURSUN M, WANG X P, WU X X. Pb0.04Co catalyst for N2O decomposition in presence of impurity gases[J]. Appl Catal B:Environ, 2016, 185:110-118. doi: 10.1016/j.apcatb.2015.12.011 [10] YU H B, WANG X P, WU X X, CHEN Y. Promotion of Ag for Co3O4 catalyzing N2O decomposition under simulated real reaction conditions[J]. Chem Eng J, 2018, 334:800-806. doi: 10.1016/j.cej.2017.10.079 [11] KIM M J, LEE S J, RYU I S, JEON M W, MOON S H, ROH H S, JEON S G. Catalytic decomposition of N2O over cobalt based spinel oxides:The role of additives[J]. Mol Catal, 2017, 422:202-207. https://www.sciencedirect.com/science/article/pii/S2468823117303048 [12] XUE L, HE H, LIU C, ZHANG C B, ZHANG B. Promotion effects and mechanism of alkali metals and alkaline earth metals on cobalt-cerium composite oxide catalysts for N2O decomposition[J]. Environ Sci Technol, 2009, 43(3):890-895. doi: 10.1021/es801867y [13] YOU Y, CHANG H, MA L, GUO L, QIN X, LI J Y, LI J H. Enhancement of N2O decomposition performance by N2O pretreatment over Ce-Co-O catalyst[J]. Chem Eng J, 2018, 347:184-192. doi: 10.1016/j.cej.2018.04.081 [14] ABUZIED B M, BAWAKED S M, KOSA S A, SCHWIEGER W. Effect of Pr, Sm, and Tb doping on the morphology, crystallite size, and N2O decomposition activity of Co3O4 nanorods[J]. J Nanomater, 2015, 56(12):1417-1423. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=Doaj000004122728 [15] ZHAO T Q, GAO Q, LI H J, XU X F. Catalytic decomposition of N2O over Y-Co3O4 composite oxides prepared by one-step hydrothermal method[J]. J Fuel Chem Technol, 2019, 47(4):446-454. doi: 10.1016/S1872-5813(19)30021-0 [16] 李岩, 邹晓玲, 张舒恒, 张相俊, 王虹, 李翠清, 宋永吉. M0.5Co2.5O4(M=La, Ce, Pr, Nd)尖晶石型复合氧化物催化剂催化分解N2O性能[J].工业催化, 2017, 25(4):28-33. doi: 10.3969/j.issn.1008-1143.2017.04.005LI Yan, ZOU Xiao-ling, ZHANG Shu-heng, ZHANG Xiang-jun, WANG Hong, LI Cui-qing, SONG Yong-ji. Catalytic decomposition of N2O on M0.5Co2.5O4(M=La, Ce, Pr, Nd) spinel oxides catalysts[J]. Ind Catal, 2017, 25(4):28-33. doi: 10.3969/j.issn.1008-1143.2017.04.005 [17] 王焘, 王虹, 李翠清, 宋永吉, 丁福臣.稀土修饰的Co/Hβ催化剂催化分解N2O[J].环境化学, 2012, 31(2):157-161. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hjhx201202004WANG Tao, WANG Hong, LI Cui-qing, SONG Yong-ji, DING Fu-chen. N2O decomposition over Co/Hβ catalysts doped with rare-earth metals[J]. Environ Chem, 2012, 31(2):157-161. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hjhx201202004 [18] ABU-ZIED B M, BAWAKED S M, KOSA S A, ALI T T, SCHWIEGER W, AQLAN F M. Effects of Nd-, Pr-, Tb-and Y-doping on the structural, textural, electrical and N2O decomposition activity of mesoporous NiO nanoparticles[J]. Appl Surf Sci, 2017, 419:399-408. doi: 10.1016/j.apsusc.2017.05.040 [19] ABU-ZIED B M, BAWAKED S M, KOSA S A, SCHWIEGER W. Impact of Gd-, La-, Nd-and Y-doping on the textural, electrical conductivity and N2O decomposition activity of CuO catalyst[J]. Int J Electrochem Sci, 2016, 11:2230-2246. [20] XUE Z W, SHEN Y S, SHEN S B, LI C L, ZHU S M. Promotional effects of Ce4+, La3+ and Nd3+ incorporations on catalytic performance of Cu-Fe-Ox for decomposition of N2O[J]. J Ind Eng Chem, 2015, 30:98-105. doi: 10.1016/j.jiec.2015.05.008 [21] LI H J, ZHENG L, ZHAO T Q, XU X F. Effect of preparation parameters on the catalytic performance of hydrothermally synthesized Co3O4 in the decomposition of N2O[J]. J Fuel Chem Technol, 2018, 46(6):717-724. doi: 10.1016/S1872-5813(18)30031-8 [22] PAN Y F, FENG M, CUI X, XU X F. Catalytic activity of alkali metal doped Cu-Al mixed oxides for N2O decomposition in the presence of oxygen[J]. J Fuel Chem Technol, 2012, 40(5):601-607. doi: 10.1016/S1872-5813(12)60024-3 [23] STELMACHOWSKI P, MANIAK G, KOTARBA A, SOJKA Z. Strong electronic promotion of Co3O4 towards N2O decomposition by surface alkali dopants[J]. Catal Commun, 2009, 10(7):1062-1065. doi: 10.1016/j.catcom.2008.12.057 [24] 郑珂, 王永钊, 胡晓波, 武瑞芳, 刘晓丽, 赵永祥.还原-氧化预处理对Co3O4催化分解N2O性能的影响[J].燃料化学学报, 2019, 47(4):455-463. http://d.old.wanfangdata.com.cn/Periodical/rlhxxb201904009ZHENG Ke, WANG Yong-zhao, HU Xu-bo, WU Rui-fang, LIU Xiao-li, ZHAO Yong-xiang. Effect of reduction-oxidation pretreatment on the catalytic performance of Co3O4 catalyst in N2O decomposition[J]. J Fuel Chem Technol, 2019, 47(4):455-463. http://d.old.wanfangdata.com.cn/Periodical/rlhxxb201904009 [25] YAO X J, CAO J, CHEN L, KANG K K, CHEN Y, TIAN M, YANG F M. Doping effect of cations (Zr4+, Al3+, and Si4+) on MnOx/CeO2 nano-rod catalyst for NH3-SCR reaction at low temperature[J]. Chin J Catal, 2019, 40(5):733-743. doi: 10.1016/S1872-2067(18)63204-8 -





 下载:
下载: