Preparation of NiO-Fe2O3/PG-γ-Al2O3 catalysts and its application in pyrolysis of biomass straw
-
摘要: 通过沉积沉淀法与均匀沉淀法制备以坡缕石与伽马氧化铝(Palygorskite-Gamma Alumina,PG-γ-Al2O3)为复合载体的负载型NiO-Fe2O3/PG-γ-Al2O3催化剂,采用了EDX、XRD、SEM、N2等温吸附-脱附等手段对催化剂进行了表征与分析。同时利用管式炉考察了NiO-Fe2O3/PG-γ-Al2O3催化剂在作物秸秆热解中的催化性能和再生使用寿命及抗积炭能力,并与两种单载体催化剂(NiO-Fe2O3/PG,NiO-Fe2O3/γ-Al2O3)进行了比较。结果表明,PG-γ-Al2O3复合载体比表面积达134.21 m2/g,平均孔径为39.65 nm。NiO-Fe2O3/PG-γ-Al2O3催化剂活性组分负载均匀,分散较好且催化剂中同时存在镍铁合金与镍铝尖晶石结构。催化剂活性测试显示,NiO-Fe2O3/PG-γ-Al2O3催化剂用于作物秸秆热解具有极高的催化活性,能够显著提高产品燃气品质、燃气中的CO与H2含量和燃气热值;相比单载体催化剂其催化活性好,再生效果佳,抗积炭能力较强。Abstract: The supported NiO-Fe2O3/Palygorskite and Gamma Alumina(NiO-Fe2O3/PG-γ-Al2O3) catalysts were prepared by deposition-precipitation and homogeneous-precipitation methods using PG-γ-Al2O3 as a carrier, and different approaches including EDX, XRD, SEM and N2 isothermal adsorption-desorption were used to characterize the synthetic catalysts. Meanwhile, the catalytic pyrolysis of biomass straw was conducted to test the catalytic activity, the regenerative service life and the anti-carbon capacity of NiO-Fe2O3/PG-γ-Al2O3 catalyst in a tube furnace, and to compare with the catalytic properties of single carrier catalysts. The results indicate that the prepared PG-γ-Al2O3 carriers have a high specific surface area of 134.21 m2/g and the average pore size is 39.65 nm. The active components are loaded uniformly and in a good dispersion over NiO-Fe2O3/PG-γ-Al2O3 catalyst, meanwhile, the Ni-Fe alloy and the nickel-aluminum spinel structure exist simultaneously in the catalyst. The catalytic activity testing shows that the NiO-Fe2O3/PG-γ-Al2O3 catalysts have a very high catalytic activity in pyrolysis of biomass straw. It could obviously improve the quality of the gas such as the content of H2 and CO and the calorific value. The catalytic activity, regeneration effect and anti-carbon deposition ability of NiO-Fe2O3/PG catalyst are better than that with single carrier catalyst.
-
Key words:
- biomass /
- pyrolysis /
- catalyst /
- regeneration
-
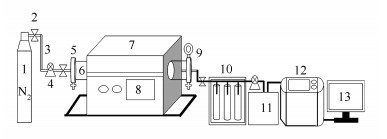
图 1 生物质热解气化反应流程示意图
Figure 1 Flow chart of the reactor for pyrolysis and gasification of biomass
1: nitrogen; 2: valve; 3: gas pipe; 4: flowmeter; 5: flange; 6: pyrolysis tube; 7: pyrolysis furnace; 8: temperature control panel; 9: pressure valve; 10: scrubbing device; 11: gas bag; 12: gas analyzer; 13: computer
表 1 秸秆的元素分析和工业分析
Table 1 Ultimate and proximate analyses of straw sample
Ultimate analysis w/% Proximate analysis w/% C H O* N S M A V FC 33.98 4.73 51.97 0.81 0.12 11.2 8.39 77.7 2.71 *: by difference 表 2 三种催化剂和PG的EDX元素分析
Table 2 EDX elemental analysis of PG and three catalysts
Catalyst Main composition and content w/% NiO Fe2O3 Al2O3 SiO2 MgO K2O TiO2 CaO MnO PG - 4.23 10.56 63.84 10.47 3.51 1.84 1.53 1.31 NFP 27.71 9.25 6.64 44.95 6.00 2.22 1.26 0.97 0.83 NFA 27.12 8.67 63.61 - 0.07 0.09 - 0.04 - NFPA 25.20 8.01 36.18 22.82 3.92 1.48 0.73 0.59 0.45 表 3 三种催化剂载体的比表面积、孔容和孔径分布
Table 3 BET surface area, pore volume, pore size of three catalyst carriers
Sample BET surface area A/(cm2·g-1) Pore volume v/(cm3·g -1) Pore diameter d/nm PG 103.36 0.119 19.29 γ-Al2O3 121.35 0.248 17.12 PA 134.21 0.325 39.65 表 4 生物质秸气化和催化气化产物产率比较
Table 4 Comparison of product yields from biomass straw pyrolysis with and without catalysts
Catalyst No catalyst NP NFP NFA NFPA Gas yield w/(m3·kg-1) 0.18 0.29 0.31 0.32 0.35 H2 production w/(g·kg-1) 5.21 8.04 9.07 10.16 11.72 QLHV/(MJ·m-3) 6.41 7.07 7.49 8.09 9.01 表 5 催化剂的C元素含量
Table 5 Carbon content of catalysts
Catalyst NFP NFA NFPA C content w/% 1.51 0.90 1.01 -
[1] ISMAIL T M, EL-SALAM M A. Parametric studies on biomass gasification process on updraft gasifier high temperature air gasification[J]. Appl Therm Eng, 2017, 112(5):1460-1473. https://www.sciencedirect.com/science/article/pii/S1359431116322232 [2] DIGMAN B, JOO H S, KIM D S. Recent progress in gasification/pyrolysis technologies for biomass conversion to energy[J]. Environ Prog Rog Sustainable Energy, 2010, 28(1):47-51. doi: 10.1002/ep.10336 [3] 胡恩源, 闫常峰, 蔡炽柳, 胡蓉荣.生物油水溶性组分的水蒸气催化重整制氢实验研究[J].燃料化学学报, 2009, 37(2):177-182. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract17424.shtmlHU En-yuan, YAN Chang-feng, CAI Chi-liu, HU Rong-rong. Experimental research on hydrogen production by catalytic steam reforming of bio-oil aqueous fraction[J]. J Fuel Chem Technol, 2009, 37(2):177-182. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract17424.shtml [4] MONTEJO C, COSTA C, RAMOS P, MÁRQUEZM D C. Analysis and comparison of municipal solid waste and reject fraction as fuels for incineration plants[J]. Appl Therm Eng, 2011, 31(13):2135-2140. doi: 10.1016/j.applthermaleng.2011.03.041 [5] BUCHIREDDY P R, BRICKA R M, RODRIGUEZ J, HOLMES W. Biomass gasification:Catalytic removal of tars over zeolites and nickel supported zeolites[J]. Energy Fuels, 2010, 24(4):2707-2715. doi: 10.1021/ef901529d [6] LI J, YAN R, XIAO B, LIANG D T. Development of nano-NiO/Al2O3 catalyst to be used for tar removal in biomass gasification[J]. Environ Sci Technol, 2008, 42(16):6224-6229. doi: 10.1021/es800138r [7] ASHOK J, KAWI S. Nickel-iron alloy supported over iron-Alumina catalysts for steam reforming of biomass tar model compound[J]. Acs Catal, 2014, 4(1):289-301. doi: 10.1021/cs400621p [8] WANG L, LI D, KOIKE M, WATANABE H. Catalytic performance and characterization of Ni-Fe catalysts for the steam reforming of tar from biomass pyrolysis to synthesis gas[J]. Appl Catal A:Gen, 2011, 392(1/2):248-255. http://www.sciencedirect.com/science/article/pii/S0016236112001159 [9] ZOU X, CHEN T, LIU H, ZHANG P. An insight into the effect of calcination conditions on catalytic cracking of toluene over 3Fe8Ni/palygorskite:Catalysts characterization and performance[J]. Fuel, 2017, 190(15):47-57. http://www.sciencedirect.com/science/article/pii/S0016236116311395 [10] 但维仪, 李建芬, 丁捷枫, 樊毅, 王强胜. NiO-Fe2O3/MD催化剂的制备及其在城市生活垃圾气化中的应用[J].燃料化学学报, 2013, 41(8):1015-1019. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18245.shtmlDAN Wei-yi, LI Jian-fen, DING Jie-feng, FAN Yi, WANG Qiang-sheng. Preparation of NiO-Fe2O3/MD catalysts and its application in gasification of municipal solid waste[J]. J Fuel Chem Technol, 2013, 41(8):1015-1019. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18245.shtml [11] 王宁, 孙自瑾, 王永钊, 高晓庆, 赵永祥. Ni-Fe/γ-Al2O3双金属催化剂的制备及其CO甲烷化性能研究[J].燃料化学学报, 2011, 39(3):219-223. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract17713.shtmlWANG Ning, SUN Zi-jin, WANG Yong-zhao, GAO Xiao-qing, ZHAO Yong-xiang. Preparation of bimetallic Ni-Fe/γ-Al2O3 catalyst and its activity for CO methanation[J]. J Fuel Chem Technol, 2011, 39(3):219-223. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract17713.shtml [12] BAMBAL A S, VECCHIO K S, CATTOLICA R J. Catalytic effect of Ni and Fe addition to gasifier bed material in the steam reforming of producer gas[J]. Ind Eng Chem Res, 2014, 53(35):13656-66. doi: 10.1021/ie502304p [13] 卜龙利, 王晓昌, 王妙刚, 周立辉.微波法制备活性炭负载金属催化剂的表征分析[J].西安建筑科技大学学报自然科学版, 2008, 40(4):532-537. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=xajz200804016&dbname=CJFD&dbcode=CJFQBO Long-li, WANG Xiao-chang, WANG Miao-gang, ZHOU Li-hui. Characteristics of carbon-supported metal catalyst prepared by microwave method[J]. J Xian Univ Arch Technol, 2008, 40(4):532-537. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=xajz200804016&dbname=CJFD&dbcode=CJFQ [14] 张玉红, 熊国兴, 盛世善, 刘盛林, 杨维慎. NiO/γ-Al2O3催化剂中NiO与γ-Al2O3间的相互作用研究[J].物理化学学报, 1999, 15(8):735-741. http://www.oalib.com/paper/4968309ZHANG Yu-hong, XIONG Guo-xing, SHENG Shi-shan, LIU Sheng-lin, YANG Wei-shen. Interaction of NiO with γ-Al2O3 supporter of NiO/γ-Al2O3 catalysts[J]. Acta Phys-Chim Sin, 1999, 15(8):735-741. http://www.oalib.com/paper/4968309 [15] AL-FATESH, A S, NAEEM M A FAKEEHA A H, ABASAEED A E. CO2 reforming of methane to produce syngas over γ-Al2O3 supported Ni-Sr catalysts[J]. Bull Chem Soc Jpn, 2013, 86(6):742-1748. doi: 10.1246/bcsj.20130002 [16] 卢雯, 孔猛, 杨琦, 范浙永, 费金华, 郑小明.载体对镍基催化剂及其甲苯水蒸气重整性能的影响[J].化学反应工程与工艺, 2012, 28(3):238-243. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=hxfy201203010&dbname=CJFD&dbcode=CJFQLU Wen, KONG Meng, YANG Qi, FAN Zhe-yong, FEI Jin-hua, ZHENG Xiao-ming. Influence of support on catalytic behavior of ni-1based catalysts in steam reforming of toluene[J].Chem React Eng Technol, 2012, 28(3):238-243. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=hxfy201203010&dbname=CJFD&dbcode=CJFQ [17] XU L, SONG H, CHOU L. Carbon dioxide reforming of methane over ordered mesoporous NiO-MgO-Al2O3 composite oxides[J]. Appl Catal B:Environ, 2011, s108-1109(6):177-190. http://www.sciencedirect.com/science/article/pii/S0926337311003973 [18] SHEN W, MOMOI H, KOMATSUBARA K, SAITO T. Marked role of mesopores for the prevention of sintering and carbon deposition in dry reforming of methane over ordered mesoporous Ni-Mg-Al oxides[J]. Catal Today, 2011, 171(1):150-155. doi: 10.1016/j.cattod.2011.04.003 [19] NEWNHAM J, MANTRI K, AMIN M H, TARDIO J. Highly stable and active Ni-1mesoporous alumina catalysts for dry reforming of methane[J]. Int J Hydrogen Energy, 2012, 37(2):1454-1464. doi: 10.1016/j.ijhydene.2011.10.036 [20] KUO H P, PAN S M, HSU H T. Comparisons of the hydrogen-1rich syngas compositions from wet rice husk slurry steam reforming reactions using different catalysts[J]. Biomass Bioenergy, 2011, 35(7):3025-3031. doi: 10.1016/j.biombioe.2011.04.005 [21] WANG L, LI D, KOIKE M, WATANABE H, XU Y. Catalytic performance and characterization of Ni-Fe catalysts for the steam reforming of tar from biomass pyrolysis to synthesis gas[J]. Appl Catal A:Gen, 2011, 392(1-2):248-255. doi: 10.1016/j.apcata.2010.11.013 [22] HOU Z Y, YASHIMA T. Meso-1porous Ni/Mg/Al catalysts for methane reforming with CO2[J]. Appl Catal A:Gen, 2004, 261(2):205-209. doi: 10.1016/j.apcata.2003.11.002 [23] ARKATOVA L A. The deposition of coke during carbon dioxide reforming of methane over intermetallides[J]. Cataly Today, 2010, 157(1/4):170-176. http://www.sciencedirect.com/science/article/pii/S0920586110001665 -





 下载:
下载: