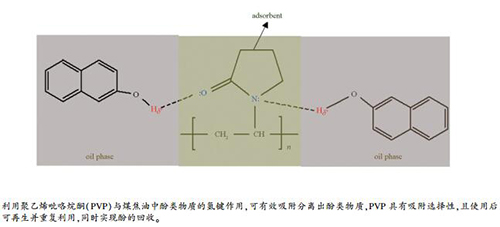
-
摘要: 煤焦油中酚类物质的有效分离,可实现其高附加值利用。针对酚类物质的分离,本研究采用聚乙烯吡咯烷酮(PVP)为吸附剂,研究了其对模型油中邻甲酚、间甲酚、对甲酚、1-萘酚和2-萘酚等的吸附性能。研究发现,PVP对酚类物质具有较大的吸附容量,其中对间甲酚、对甲酚、1-萘酚和2-萘酚的最大吸附量均可达1000 mg/g以上。同时发现,PVP上的Lewis碱性位点(C=O和C-N)可与酚羟基之间形成氢键作用,该作用的强度受酚类物质空间位阻影响。PVP具有一定的吸附选择性,在苯并呋喃或喹啉存在下,依然能够有效吸附2-萘酚。此外,使用过的PVP可再生并重复利用,同时实现酚的回收。可见,PVP是一种可用于分离煤焦油中酚类物质的优良吸附剂。Abstract: Effective separation of phenols in coal tar is essential for enhancing its application value. In this work, polyvinylpyrrolidone (PVP) was used as a sorbent in the separation of phenols in model oils; the adsorption performance of PVP towards o-cresol, m-cresol, p-cresol, 1-naphthol, and 2-naphthol was then comparatively investigated. The results indicate that PVP possesses high adsorption capacity towards the phenols; the maximum adsorbance of PVP towards m-cresol, p-cresol, 1-naphthol, and 2-naphthol is higher than 1000 mg/g. For the adsorption of phenols on PVP, H-bonds are formed between the Lewis basic sites (C=O and N) of PVP and the phenolic -OH group and the H-bonding intensity is influenced by the steric hindrance of phenols. Furthermore, PVP shows high adsorption selectivity; 2-naphthol can be adsorbed effectively on PVP even in the presence of benzofuran or quinoline. Moreover, PVP can be regenerated for recycling where phenols are recovered as well. As a result, PVP is a promising sorbent for the separation of phenols from the coal tar oil.
-
Key words:
- polyvinylpyrrolidone /
- coal tar /
- phenols /
- adsorption
-
表 1 酚类物质的吸附等温线模型参数
Table 1 Adsorption isotherm model parameters of various phenols on PVP
Phenol Langmuir Freundlich Qm b R2 KF 1/n R2 o-cresol 892 1.74×10-4 0.995 9.865 0.429 0.940 m-cresol 1437 5.12×10-5 0.996 2.012 0.591 0.983 p-cresol 1334 7.85×10-5 0.986 2.863 0.572 0.980 1-naphthol 1122 2.06×10-4 0.996 9.983 0.463 0.928 2-naphthol 1603 1.15×10-4 0.994 9.281 0.479 0.954 -
[1] YAO C F, HOU Y C, REN S H, WU W Z, JI Y A, LIU H. Sulfonate based zwitterions:A new class of extractants for separating phenols from oils with high efficiency via forming deep eutectic solvents[J]. Fuel Process Technol, 2018, 178:206-212. doi: 10.1016/j.fuproc.2018.05.031 [2] JIAO T T, GONG M M, ZHUANG X L, LI C S, ZHANG S J. A new separation method for phenolic compounds from low-temperature coal tar with urea by complex formation[J]. J Ind Eng Chem, 2015, 29:344-348. doi: 10.1016/j.jiec.2015.04.013 [3] PANG K, HOU Y C, WU W Z, GUO W J, PENG W, MASH N. Kenneth efficient separation of phenols from oils via forming deep eutectic solvents[J]. Green Chem, 2012, 14(9):2398-2401. doi: 10.1039/c2gc35400d [4] 刘贞贞, 骆仲泱, 马帅, 方梦祥.煤焦油中酚类物质对焦油组分加氢脱氮脱硫以及芳烃饱和的影响[J].燃料化学学报, 2015, 43(11):1327-1333. doi: 10.3969/j.issn.0253-2409.2015.11.007LIU Zhen-zhen, LUO Zhong-yang, MA Shuai, FANG Meng-xiang. Influence of phenolics on hydrodenitrogenation, hydrodesulfurization and hyrodearomatization of coal tar components[J]. J Fuel Chem Technol, 2015, 43(11):1327-1333. doi: 10.3969/j.issn.0253-2409.2015.11.007 [5] JI Y A, HOU Y C, REN S H, YAO C F, WU W Z. Separation of phenolic compounds from oil mixtures using environmentally benign biological reagents based on Brønsted acid-Lewis base interaction[J]. Fuel, 2019, 239:926-934. doi: 10.1016/j.fuel.2018.11.007 [6] ZAHOOR M, MAHRAMANLIOGLU M. Removal of phenolic substances from water by adsorption and adsorption-ultrafiltration[J]. Sep Sci Technol, 2011, 46(9):1482-1494. doi: 10.1080/01496395.2011.561269 [7] SCHHBERT H H, SONG C. Chemicals and materials from coal in the 21st century[J]. Fuel, 2002, 81(1):15-32. doi: 10.1016/S0016-2361(00)00203-9 [8] GAO J J, DAI Y F, MA W Y, XU H H, LI C X. Efficient separation of phenol from oil by acid-base complexing adsorption[J]. Chem Eng J, 2015, 281:749-758. doi: 10.1016/j.cej.2015.06.099 [9] PATEL R N, BANDYOPADHYAY S, GANESH A. Extraction of cardanol and phenol from bio-oils obtained through vacuum pyrolysis of biomass using supercritical fluid extraction[J]. Energy, 2011, 36(3):1535-1542. doi: 10.1016/j.energy.2011.01.009 [10] BHADRA B N, AHMED I, JHUNG S H. Remarkable adsorbent for phenol removal from fuel:Functionalized metal-organic framework[J]. Fuel, 2016, 174:43-48. doi: 10.1016/j.fuel.2016.01.071 [11] MENG H, GE C T, REN N N, MA W Y, LU Y Z, LI C X. Complex extraction of phenol and cresol from model coal tar with polyols, ethanol amines, and ionic liquids thereof[J]. Ind Eng Chem Res, 2013, 53(1):355-362. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=849ea00d2d460770d550139ceada169d [12] YAO C F, HOU Y C, REN S H, WU W Z, ZHANG K, JI Y A, LIU H. Efficient separation of phenol from model oils using environmentally benign quaternary ammonium-based zwitterions via forming deep eutectic solvents[J]. Chem Eng J, 2017, 326:620-626. doi: 10.1016/j.cej.2017.06.007 [13] LIN K L, PAN J Y, CHEN Y W, CHENG R M, XU X C. Study the adsorption of phenol from aqueous solution on hydroxyapatite nanopowders[J]. J Hazard Mater, 2009, 161(1):231-40. doi: 10.1016/j.jhazmat.2008.03.076 [14] 孙爽, 李未康, 张娟, 赵地顺.聚合离子液体的吸附分离应用研究进展[J].现代化工, 2017, 37(6):38-42. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=xdhg201706009SUN Shuang, LI Wei-kang, ZHANG Juan, ZHAO Di-shun. Research progress on application of polymeric ionic liquids in adsorption separation[J]. Mod Chem Ind, 2017, 37(6):38-42. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=xdhg201706009 [15] ARCHANA V, MEERA S, BEGUM K M, ANANTHARAMAN N. Studies on removal of phenol using ionic liquid immobilized polymeric micro-capsules[J]. Arabian J Chem, 2016, 9(3):371-382. doi: 10.1016/j.arabjc.2013.03.017 [16] XIE K, SHAN C H, QI J S, QIAO S, ZENG Q S, ZHANG L Y. Study of adsorptive removal of phenol by MOF-5[J]. Desalin Water Treat, 2014, 54(3):654-659. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=5d47dcbc51205ffb8d43bdd987e5b539 [17] KOCZKUR M K, STAFANOS M, LAKSHMINARAYANA P, SARA E S. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis[J]. Dalton Trans, 2015, 44(41):17883-17905. doi: 10.1039/C5DT02964C [18] AYNUR O A, TATIANA I N, IVA V V, MARK T C, TERRY W S, RAIPH K, GERRIT S. Multivariate discrimination between modes of toxic action of phenols[J]. Qsar Comb Sci, 2015, 21(1):12-22. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=a6621cb3df7a092b55cfbd6a619059bb [19] CHEN B L, CHEN Z M. Sorption of naphthalene and 1-naphthol by biochars of orange peels with different pyrolytic temperatures[J]. Chemosphere, 2009, 76(1):123-133. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=50d9dd6097238a985d6f8ff0df6744cc [20] ROSEN M J, LI F. The adsorption of gemini and conventional surfactants onto some soil solids and the removal of 2-naphthol by the soil surfaces[J]. J Colloid Interface Sci, 2001, 234(2):418-424. doi: 10.1006/jcis.2000.7293 [21] WANG Y, HOU Y C, WU W Z, LIU D D, JI Y A, REN S H. Roles of hydrogen bond donor and hydrogen bond acceptor in the extraction of toluene from n-heptane using deep eutectic solvents[J]. Green Chem, 2016, 10:3089-3097. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=623ef72d9cf23aaa87218d69de48e5ea [22] 韩鹏飞, 赵红, 董旭峰, 谭锁奎, 齐民, 纪松.电喷法钛氧/聚乙烯吡咯烷酮/硬脂酸颗粒及其电流变性能[J].功能材料, 2017, 48(1):1135-1138. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=gncl201701023HAN Peng-fei, ZHAO Hong, DONG Xu-feng, TAN Suo-kui, QI Min, JI Song. Effect of etching process on fast-epitaxial SiC thick films[J]. J Funct Mater, 2017, 48(1):1135-1138. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=gncl201701023 [23] DUAN Z B, BU T T, BIAN H, ZHU L J, XIANG Y Z, XIA D D. Effective removal of phenylamine, quinoline and indol from light oil by β-cyclodextrin aqueous solution through molecular inclusion[J]. Energy Fuels, 2018, 32(9):9280-9288. doi: 10.1021/acs.energyfuels.8b02086 [24] 甘五鹏, 王红心.活性炭吸附法脱除废水中的苯酚及吸附剂再生的研究[J].辽宁化工, 1999, 28(6):337-339. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=lnhg199906010GAN Wu-peng, WANG Hong-xin. Study on activated carbon adsorption to remove phenol from wastewater and regeneration of the adsorbent[J]. Liaoling Chem Ind, 1999, 28(6):337-339. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=lnhg199906010 [25] ZENG Q C, WU D C, ZHOU C, XU F, FU R W, LI Z H, LIANG Y R, SU D S. Template-free fabrication of hierarchical porous carbon based on intra-/inter-sphere crosslinking of monodisperse styrene-divinylbenzene copolymer nanospheres[J]. Chem Commun, 2010, 46(32):5927-5929. doi: 10.1039/c0cc00449a -





 下载:
下载: