Preparation and characterization of hydrolysis component γ-Ga2O3 for hydrogen production by low temperature steam reforming of dimethyl ether in slurry reactor
-
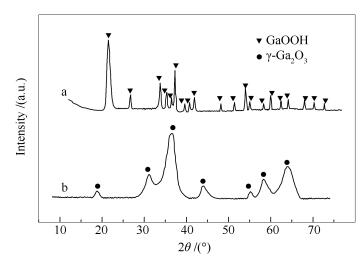
摘要: 以有机溶剂均匀沉淀法制备了镓的氧化物,借助XRD、NH3-TPD、TEM和BET等手段对物相结构、表面性质等进行了表征。结果表明,制备过程中得到的前驱体为GaOOH,前驱体经500 ℃热处理后得到γ-Ga2O3。γ-Ga2O3的晶格类型与γ-Al2O3相似,为有阳离子缺陷的立方尖晶石结构。表面具有酸量较大的中强酸中心,而弱酸中心含量相对较少。微观上大多为厚10 nm、直径100 nm左右的二维纳米片,大部分纳米片分布于一个方向,一些组成花瓣形。将制得的γ-Ga2O3用于DME水解反应,结果表明,270 ℃下DME的转化率可达24%,接近平衡转化率,反应后催化剂的织构性质没有显著变化,比表面积仍可达到130 m2/g。将γ-Ga2O3与Cu基催化剂复合后用于270 ℃下的低温浆态床DME水蒸气重整反应,DME转化率和H2选择性高达99%和68%,经200 h反应后催化剂仍能保持95%以上的活性,表现出良好的工业化应用前景。Abstract: The oxide of gallium was prepared by homogeneous precipitation in organic solvent. The physical structure and surface properties were characterized by XRD, NH3-TPD, TEM and BET. The results reveal that γ-Ga2O3 was obtained after 500 ℃ heat treatment of the precursor of GaOOH. The lattice type of the γ-Ga2O3 is similar to that of γ-Al2O3, which belongs to cubic, cation-deficient spinel structure. The γ-Ga2O3 has a moderate acid center with larger amount of acid content. Most of the γ-Ga2O3 is two-dimensional nanoflakes with thickness of 10 nm and diameter of 100 nm. These nanoflakes are mainly distributed in one direction, and some of them form petal-shaped patterns. The experimental results showed that the conversion rate of dimethyl ether (DME) was about 24% at 270 ℃, which was close to equilibrium conversion. The texture properties of the catalyst had no significant change after the reaction and the specific surface of the catalyst was about 130 m2/g. The composite catalyst composed of the γ-Ga2O3 and Cu-based catalyst was used to DME steam reforming reaction in slurry bed at 270 ℃. The conversion of DME and selectivity of H2 were as high as about 99% and 68%, respectively. The conversion of DME was over 95% after reaction time of 200 h.
-
Key words:
- homogeneous precipitation /
- γ-Ga2O3 /
- low temperature /
- slurry bed /
- dimethyl ether /
- steam reforming
-
表 1 催化剂的织构性质
Table 1 Texture properties of the catalyst
Catalyst ABET/
(m2·g-1)Pore diameter
d/nmPore volume
v/ (cm3·g-1)Fresh γ-Ga2O3 131.2 8.24 0.46 Used γ-Ga2O3 128.7 8.13 0.45 -
[1] FABI V, NICOLI M V D, SPIGLIANTINI G, CORGNATI S P. Insights on pro-environmental behavior towards post-carbon society[J]. Energy Procedia, 2017, 134:462-469. doi: 10.1016/j.egypro.2017.09.604 [2] ABBASI T, PREMALATHA M, ABBASI S A. The return to renewables:Will it help in global warming control[J]. Renewable Sustainable Energy Rev, 2011, 15(1):891-894. doi: 10.1016/j.rser.2010.09.048 [3] KIRSCHKE S, NEWIG J, VÖLKER J, BORCHARDT D. Does problem complexity matter for environmental policy delivery? How public authorities address problems of water governance[J]. J Environ Manage, 2017, 196:1-7. doi: 10.1016/j.jenvman.2017.02.068 [4] FOLEY D K, REZAI A, TAYLOR L. The social cost of carbon emissions:Seven propositions[J]. Econom Lett, 2013, 121(1):90-97. doi: 10.1016/j.econlet.2013.07.006 [5] SOUCHE I, CHATALIC A, BREGEON B G. Hydrogen combustion characteristics, its performances as a clean fuel compared to fossil fuel ones[J]. Int J Hydrogen Energy, 1988, 13(5):299-310. doi: 10.1016/0360-3199(88)90054-7 [6] WINTER C J. Hydrogen energy-Abundant, efficient, clean:A debate over the energy-system-of-change[J]. Int J Hydrogen Energy, 2009, 34(14):S1-S52. doi: 10.1016/j.ijhydene.2009.05.063 [7] BICELLI L P. Hydrogen:A clean energy source[J]. Int J Hydrogen Energy, 1986, 11(9):555-562. doi: 10.1016/0360-3199(86)90121-7 [8] SEMELSBERGER T A, BORUP R L, GREENE H L. Dimethyl ether (DME) as an alternative fuel[J]. J Power Sources, 2006, 156(2):497-511. doi: 10.1016/j.jpowsour.2005.05.082 [9] SEMELSBERGER T A, BORUP R L. Thermodynamic equilibrium calculations of hydrogen production from the combined processes of dimethyl ether steam reforming and partial oxidation[J]. J Power Sources, 2006, 155(2):340-352. doi: 10.1016/j.jpowsour.2005.04.031 [10] FAUNGNAWAKIJ K, VIRIYA-EMPIKUL N, TANTHAPANICHAKOON W. Evaluation of the thermodynamic equilibrium of the autothermal reforming of dimethyl ether[J]. Int J Hydrogen Energy, 2011, 36(10):5865-5874. doi: 10.1016/j.ijhydene.2011.02.027 [11] GALVITA V V, SEMIN G L, BELYAEV V D, YURIEVA T M, SOBYANIN V A. Production of hydrogen from dimethyl ether[J]. Appl Catal A:Gen, 2001, 216(1):85-90. [12] SOBYANIN V A, CAVALLARO S, FRENI S. Dimethyl ether steam reforming to feed molten carbonate fuel cells[J]. Appl Catal A:Gen, 2000, 14(6):1139-1142. https://www.deepdyve.com/lp/elsevier/hydrogen-production-for-fuel-cells-via-steam-reforming-of-dimethyl-YGTaZ3islM [13] 冯冬梅. 二甲醚重整制氢催化反应过程的研究[D]. 北京: 清华大学, 2008.FENG Dong-mei. Research on the catalytic process of hydrogen production from dimethyl ether reforming[D]. Beijing: Tsinghua University, 2008. [14] SEMELSBERGER T A, OTT K C, BORUP R L, GREENE H L. Role of acidity on the hydrolysis of dimethyl ether (DME) to methanol[J]. Appl Catal B:Environ, 2005, 61(3/4):281-287. https://www.sciencedirect.com/science/article/pii/S0926337305002687?_escaped_fragment_= [15] SEMELSBERGER T A, OTT K C, BORUP R L, GREENE H L. Generating hydrogen-rich fuel-cell feeds from dimethyl ether (DME) using Cu/Zn supported on various solid-acid substrates[J]. Appl Catal A:Gen, 2006, 309(2):210-223. doi: 10.1016/j.apcata.2006.05.009 [16] YANG M, MEN Y, LI S L, CHEN G W. Enhancement of catalytic activity over TiO2-modified Al2O3 and ZnO-Cr2O3 composite catalyst for hydrogen production via dimethyl ether steam reforming[J]. Appl Catal A:Gen, 2012, 433-434(31):26-34. [17] 李娟, 海航, 闫常峰, 胡蓉蓉, 么志伟, 罗伟民, 郭常青, 李文博.焙烧温度对二甲醚水蒸气重整制氢Cu/ZnO/Al2O3/Cr2O3+H-ZSM-5双功能催化剂性能的影响[J].燃料化学学报, 2012, 40(10):1240-1245. http://rlhxxb.sxicc.ac.cn/CN/Y2012/V40/I10/1240LI Juan, HAI Hang, YAN Chang-feng, HU Rong-rong, YAO Zhi-wei, LUO Wei-min, GUO Chang-qing, LI Wen-bo. Effect of calcination temperature on properties of Cu/ZnO/Al2O3/Cr2O3+H-ZSM-5 bi-functional catalysts for steam reforming of dimethyl ether[J]. J Fuel Chem Technol, 2012, 40(10):1240-1245. http://rlhxxb.sxicc.ac.cn/CN/Y2012/V40/I10/1240 [18] 贺建平, 张磊, 陈琳, 杨占旭, 佟宇飞. CeO2改性Cu/Zn-Al水滑石衍生催化剂对甲醇水蒸气重整制氢性能的影响[J].高等学校化学学报, 2017, 38(10):1822-1828.HE Jian-ping, ZHANG Lei, CHEN Lin, YANG Zhan-xu, TONG Yu-fei, Effect of CeO2 on Cu/Zn-Al catalysts derived from hydrotalcite precursor for methanol steam reforming[J]. Chem J Chin Univ, 2017, 38(10):1822-1828. [19] 王东升, 谭猗生, 韩怡卓, 椿范立.浆态床合成二甲醚复合催化剂失活原因探索[J].燃料化学学报, 2008, 36(2):176-180. http://rlhxxb.sxicc.ac.cn/EN/Y2008/V36/I02/176WANG Dong-sheng, TAN Yi-sheng, HAN Yi-zhuo, Noritatsu TSUBAKI. Study on deactivation of hybrid catalyst for dimethyl ether synthesis in slurry reactor[J]. J Fuel Chem Technol, 2008, 36(2):176-180. http://rlhxxb.sxicc.ac.cn/EN/Y2008/V36/I02/176 [20] 高志华, 黄伟, 李俊芳, 阴丽华, 谢克昌.以拟薄水铝石为铝源制备浆态床二甲醚合成催化剂[J].高等学校化学学报, 2009, 30(3):534-538. http://cdmd.cnki.com.cn/Article/CDMD-10112-2008131185.htmGAO Zhi-hua, HUANG Wei, LI Jun-fang, YIN Li-hua, XIE Ke-chang. Liquid-phase preparation of DME slurry catalysts using pseudo-boehmite as aluminum source[J]. Chem J Chin Univ, 2009, 30(3):534-538. http://cdmd.cnki.com.cn/Article/CDMD-10112-2008131185.htm [21] FAUNGNAWAKIJ K, FUKUNAGA T, KIKUCHI R, EGUCHI K. Deactivation and regeneration behaviors of copper spinel-alumina composite catalysts in steam reforming of dimethyl ether[J]. J Catal, 2008, 256(1):37-44. doi: 10.1016/j.jcat.2008.02.022 [22] TENG Y, SONG L X, PONCHEL A, YANG Z K, XIA J. Self-assembled metastable γ-Ga2O3 nanoflowers with hexagonal nanopetals for solar-blind photodetection[J]. Adv Mater, 2014, 26(36):6238-6243. doi: 10.1002/adma.201402047 [23] NAKATANI T, WATANABE T, TAKAHASHI M, MIYAHARA Y, DEGUCHI H, IWAMOTO S, KANAI H, INOUE M. Characterization of γ-Ga2O3-Al2O3 prepared by solvothermal method and its performance for methane-SCR of NO[J]. J Phys Chem A, 2009, 113(25):7021-7029. doi: 10.1021/jp901569s [24] PLAYFORD H Y, HANNON A C, TUCKER M G, DAWSON D M, ASHBROOK S E, KASTIBAN R J, SLOAN J, WALTON R I. Characterisation of structural disorder in γ-Ga2O3[J]. J Phys Chem C, 2014, 118(29):16188-16198. doi: 10.1021/jp5033806 [25] 杨锡尧.固体催化剂的研究方法-第十三章程序升温技术(下)[J].石油化工, 2002, 31(1):63-73. http://industry.wanfangdata.com.cn/dl/Detail/Periodical?id=Periodical_syhg200201017YANG Xi-yao. Methods for the investigation of solid catalyst-Chapter 13, Temperature programming analytical technique (Part 2)[J]. Petrochem Technol, 2002, 31(1):63-73. http://industry.wanfangdata.com.cn/dl/Detail/Periodical?id=Periodical_syhg200201017 [26] 周迎春, 李昊, 张启俭, 马畅.二甲醚蒸汽重整制氢PdZn系催化剂[J].石油学报(石油加工), 2011, 27(4):537-542. http://cdmd.cnki.com.cn/Article/CDMD-10141-1013197470.htmZHOU Ying-chun, LI Hao, ZHANG Qi-jian, MA Chang. PdZn-based catalysts for steam reform ing of dimethyl ether[J]. Acta Pet Sin (Pet Process), 2011, 27(4):537-542. http://cdmd.cnki.com.cn/Article/CDMD-10141-1013197470.htm [27] 寇素原, 王晓蕾, 任克威, 潘相敏, 林瑞, 马建新.二甲醚水蒸气重整制氢过程的热力学分析[J].天然气化工, 2009, 34(1):35-40. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=nyjxxb201309001KOU Su-yuan, WANG Xiao-lei, REN Ke-wei, PAN Xiang-min, LI Rui, MA Jian-xin. Thermodynamic analysis of hydrogen production from dimethyl ether steam reforming[J]. Nat Gas Chem Ind, 2009, 34(1):35-40. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=nyjxxb201309001 [28] TAKEISHI K, AKAIKE Y. Hydrogen production by dimethyl ether steam reforming over copper alumina catalysts prepared using the sol-gel method[J]. Appl Catal A:Gen, 2016, 510:20-26. doi: 10.1016/j.apcata.2015.09.027 -





 下载:
下载: