Transformation of nitrogen during pyrolysis of Na-loaded Shengli brown coal
-
摘要: 采用具有流化床/固定床特征的石英反应器进行不同Na负载量的胜利褐煤热解实验,考察不同Na负载量对煤热解过程中氮迁移的影响。用紫外可见分光光度计分析气相的NH3和HCN,用X光电子能谱仪(XPS)表征固体半焦中有机氮的存在形式。结果表明,低温热解时,增加Na的含量对煤中氮转化为NH3起促进作用;高温热解时,Na抑制煤中氮转化为NH3。Na对煤中氮转化为HCN表现为抑制作用,这种影响规律不随温度而变化。载Na量增加降低半焦氮含量,促进半焦中季氮的生成,但这种影响在低温时不明显。Abstract: The impact of Na content on nitrogen transformation during the pyrolysis of Shengli raw coal and the Na-loaded coal in a fixed-bed/fluidized-bed quartz reactor was investigated. The quantification of NH3 and HCN in gas product was carried out using an ultraviolet-visible spectrophotometer while the occurrence modes of nitrogen in the solid chars were detected by X-ray photoelectron spectroscopy (XPS). The results indicate that the transformation of coal-N to NH3 can be catalytically enhanced by certain amount of Na at low temperature. When the final pyrolysis temperature is relatively high, the presence of Na appears to inhibit the formation of NH3. Meanwhile, for any given pyrolysis temperature, the production of HCN will be suppressed by Na. When the pyrolysis temperature is high, the increase of Na content in coal causes the reduction of nitrogen remaining in char and promotes quaternary nitrogen formation, whereas, the effect is negligible at low temperature.
-
Key words:
- brown coal /
- pyrolysis /
- Na /
- nitrogen
-
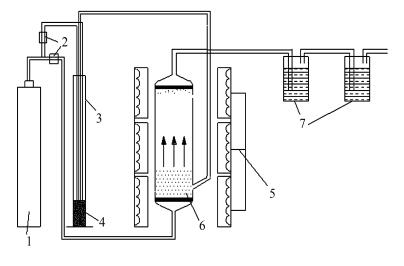
图 1 流化床/固定床反应器热解实验装置流程示意图[18]
Figure 1 Schematic diagram of coal pyrolysis reaction system
1: argon; 2: flow rate controller; 3: coal feeder; 4: coal; 5: electrical furnace; 6: fluidized-bed/fixed-bed reactor; 7: NaOH or H2SO4 solution in absorption bottles
表 1 煤样的工业分析和元素分析
Table 1 Proximate and ultimate analysis of coal samples
Coal sample Proximate analysis w/% Ultimate analysis wdaf/% Mad Ad Vdaf FCdaf C H O* N S Na0 6.44 8.54 45.93 54.07 60.55 4.66 33.28 0.95 0.56 Na1 6.08 9.73 40.57 59.43 60.38 5.11 32.74 1.18 0.59 Na2 6.30 10.91 41.09 58.91 60.37 4.97 33.04 1.15 0.47 Na3 6.76 11.7 41.39 58.61 59.60 5.01 33.92 1.15 0.44 Na6 13.07 17.77 35.90 64.10 50.64 5.12 42.72 1.10 0.42 *: by difference;Na0, Na1, Na2, Na3 and Na6 represent coal loaded with 1%Na, 2%Na, 3%Na and 6%Na respectively 表 2 煤样的碱金属及碱土金属分析
Table 2 Alkali and alkaline earth metal analysis of coal samples
Coal sample Content w/% Na Ca Mg Na0 1.51 0.563 0.137 Na1 1.99 0.555 0.280 Na2 3.07 0.660 0.173 Na3 3.85 0.728 0.334 Na6 6.37 0.761 0.347 表 3 不同氮形态的结合能
Table 3 Binding energy of different nitrogen morphologies
Nitrogen morphology Binding energy E/eV Pyridine-N 398.7±0.4 Pyrrole-N 400.5±0.3 Quaternary-N 401.1±0.3 表 4 不同Na含量煤1 073 K热解半焦中氮官能团的相对含量
Table 4 Distribution (relative percentage) of nitrogen functional groups in chars from pyrolysis of coal with different sodium contents at 1 073 K
Char Content w/% N-6 N-5 N-Q Na0 char 33.43 55.69 10.87 Na1 char 33.59 53.14 13.27 Na2 char 32.84 52.83 14.33 Na3 char 24.82 42.77 32.41 -
[1] WU Z H, SUGIMOTO Y, KAWASHIMA H. The influence of mineral matter and catalyst on nitrogen release during slow pyrolysis of coal and related material:A comparative study[J]. Energy Fuels, 2002, 16(2):451-456. doi: 10.1021/ef010183q [2] FRIEBEL J, KÖPSEL R F. The fate of nitrogen during pyrolysis of German low rank coals-a parameter study[J]. Fuel, 1999, 78(8):923-932. doi: 10.1016/S0016-2361(99)00008-3 [3] WU Z H, SUGIMOTO Y, KAWASHIMA H. Effect of demineralization and catalyst addition on N2 formation during coal pyrolysis and on char gasification[J]. Fuel, 2003, 82(15/17):2057-2064. http://www.sciencedirect.com/science/article/pii/S001623610300187X [4] TSUBOUCHI N, OHTSUKA Y. Formation of N2 during pyrolysis of Ca-loaded coals[J]. Fuel, 2002, 81(11/12):1423-1431. https://www.mysciencework.com/publication/show/e86ecee908767991c6038d697097b963 [5] OHTSUKA Y, WU Z, EDWARD F. Effect of alkali and alkaline metals on nitrogen release during temperature programmed pyrolysis of coal[J]. Fuel, 1997, 76(14/15):1361-1367. https://www.researchgate.net/publication/263600839_Effect_of_alkali_and_alkaline_earth_metals_on_nitrogen_release_during_temperature_programmed_pyrolysis_of_coal [6] 胡俊豪, 黎阳, 杨海平, 杨晴, 邵敬爱, 王贤华, 陈汉平.煤热解过程中含氮产物的析出及金属离子的催化特性研究[J].燃料化学学报, 2014, 42(8):913-921. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18465.shtmlHU Jun-hao, LI Yang, YANG Hai-ping, YANG Qing, SHAO Jing-ai, WANG Xian-hua, CHEN Han-ping. Release of nitrogenous products and the catalytic characteristics of metal ions during coal pyrolysis[J]. J Fuel Chem Technol, 2014, 42(8):913-921. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18465.shtml [7] 孟丽莉, 付春慧, 王美军, 常丽萍.碱金属碳酸盐对褐煤程序升温热解过程中H2S和NH3生成的影响[J].燃料化学学报, 2012, 40(2):138-142. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract17874.shtmlMENG Li-li, FU Chun-hui, WANG Mei-Jun, CHANG Li-ping. Effect of alkali carbonates on the formation of H2S and NH3 during temperature programmed pyrolysis of brown coal[J]. J Fuel Chem Technol, 2012, 40(2):138-142. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract17874.shtml [8] 赵宗彬, 李文, 李保庆, 陈皓侃.钠、钙、铁对模型化合物热解及燃烧过程中氮逸出规律的影响[J].燃料化学学报, 2002, 30(4):294-299. http://www.cnki.com.cn/Article/CJFDTOTAL-RLHX200204001.htmZHAO Zong-bin, LI Wen, LI Bao-qing, CHEN Hao-kan. Effect of Na, Ca and Fe on evolution of fuel-nitrogen during pyrolysis and combustion of model compound[J]. J Fuel Chem Technol, 2002, 30(4):294-299. http://www.cnki.com.cn/Article/CJFDTOTAL-RLHX200204001.htm [9] ZHAO Z B, LI W, QIU J S, LI B Q. Effect of Na、Ca and Fe on the evolution of nitrogen species during pyrolysis and combustion of model chars[J]. Fuel, 2003, 82(15/17):1839-1844. [10] 秦玲丽. 金属化合物对煤热解过程中氮、硫转化的影响[D]. 太原: 太原理工大学, 2007.QIN Ling-li. Effect of metal compound on the transformation of coal-N and coal-S during coal pyrolysis[D]. Taiyuan:Taiyuan University of Technology, 2007. [11] 王永刚, 郑盼盼, 杨萨莎, 张书, 白艳萍, 贾晓璐.酸洗脱矿对胜利褐煤热解过程中N迁移转化的影响[J].燃料化学学报, 2014, 42(5):519-526. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18404.shtmlWANG Yong-gang, ZHENG Pan-pan, YANG Sa-sha, BAI Yan-ping, JIA Xiao-lu. Influence of demineralization using acid wash on N migration and transformation during pyrolysis of Shengli brown coal[J]. J Fuel Chem Technol, 2004, 42(5):519-526. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18404.shtml [12] 许慎启, 周志杰, 代正华, 于广锁, 龚欣.碱金属及灰分对煤焦炭微晶结构及气化反应特性的影响[J].高校化学工程学报, 2010, 24(1):64-70. http://www.cnki.com.cn/Article/CJFDTOTAL-GXHX201001013.htmXU Shen-qi, ZHOU Zhi-jie, DAI Zheng-hua, YU Guang-suo, GONG Xin. Effects of alkalimetal and ash on crystallite structure of coal char during pyrolysis and on gasification reactivity[J]. J Chem Eng Chin Univ, 2010, 24(1):64-70. http://www.cnki.com.cn/Article/CJFDTOTAL-GXHX201001013.htm [13] LI C Z. Some recent advances in the understanding of the pyrolysis gasification behavior of Victorian brown coal[J]. Fuel, 2007, 86(12/13):1664-1683. https://www.researchgate.net/publication/222034353_Some_recent_advances_in_the_understanding_of_the_pyrolysis_and_gasification_behaviour_of_Victorian_brown_coal [14] WOOD B J, SANCIER K M. The mechanism of the catalytic gasification of coal char:A critical review[J]. Catal Rev, 1984, 26(2):233-279. doi: 10.1080/01614948408078065 [15] VERNAGLIA B A, WORNAT M J, LI C Z, NELSON P F. The effects of pyrolysis temperature and ion-exchanged metals on the composition of brown coal tars produced in a fluidized-bed reactor[J]. Symp Combust, 1996, 26(2):3287-3294. doi: 10.1016/S0082-0784(96)80175-5 [16] 李春柱.维多利亚褐煤褐煤科学进展[M].北京:化学工业出版社, 2009.LI Chun-zhu. Advance in the Science of Victorian Brown Coal[M]. Beijing:Chemical Industry Press, 2009. [17] ZHANG S, HAYASHI J I, LI C Z. Volatilisation and catalytic effects of alkali and alkaline earth metallic species during the cytolysis and gasification of Victorian brown coal. Part IX. Effects of volatile-char interactions on char-H2O and char-O2 reactivities[J].Fuel, 2011, 90(4):1655-1661. doi: 10.1016/j.fuel.2010.11.008 [18] 张书, 白艳萍, 米亮, 郑盼盼, 陈绪军, 许德平, 王永刚.升温速率对胜利褐煤热解过程中N迁移转化的影响[J].燃料化学学报, 2013, 41(10):1153-1159. doi: 10.1016/S1872-5813(13)60048-1ZHANG Shu, BAI Yan-ping, MI Liang, ZHENG Pan-pan, CHEN Xu-jun, XU De-ping, WANG Yong-gang. Effect of heating rate on the migration and transformation of N during pyrolysis of Shengli brown coal[J]. J Fuel Chem Technol, 2013, 41(10):1153-1159. doi: 10.1016/S1872-5813(13)60048-1 [19] 秦玲丽, 崔银萍, 徐明艳, 常丽萍.煤氮催化转化研究中的主要影响因素分析[J].现代化工, 2006, 26(2):382-385. http://www.cnki.com.cn/Article/CJFDTOTAL-XDHG2006S2106.htmQIN Ling-li, CUI Yin-ping, XU Ming-yan, CHANG Li-ping. Main influencing factors in the, research on catalytic conversion of coal-nitrogen[J]. Mod Chem Ind, 2006, 26(2):382-385. http://www.cnki.com.cn/Article/CJFDTOTAL-XDHG2006S2106.htm [20] MANZOORI A R, AGARWAL P K. The fate of organically bound inorganic elements and sodium chloride during fluidized bed combustion of high sodium, high sulphur low rank coals[J]. Fuel, 1992, 71(5):513-522. doi: 10.1016/0016-2361(92)90148-H [21] YAN X, CHE D F, XU T M. Effect of rank, temperature and inherent minerals on nitrogen emissions during coal pyrolysis in a fixed bed reactor[J]. Fuel Process Technol, 2005, 86(7):739-756. doi: 10.1016/j.fuproc.2004.08.005 [22] 闫晓, 车得福, 徐通模.煤热解过程中焦炭氮变化规律的试验研究[J].西安交通大学学报, 2004, 38(9):980-984. http://www.cnki.com.cn/Article/CJFDTOTAL-XAJT200409024.htmYAN Xiao, CHE De-fu, XU Tong-mo. Experimental investigation on char nitrogen conversion during coal pyrolysis[J]. J Xi'an Jiaotong Univ, 2004, 38(9):980-984. http://www.cnki.com.cn/Article/CJFDTOTAL-XAJT200409024.htm [23] LI C Z, NELSON P F. Interaction of quarts, zircon sand and stainless steel with ammonia implications for the measurement of ammonia at high temperature[J]. Fuel, 1996, 75(4):525-526. doi: 10.1016/0016-2361(95)00256-1 [24] 张双全.煤化学[M]. 2版.徐州:中国矿业大学出版社, 2010.ZHANG Shuang-quan. Coal Chemistry[M]. 2nd ed. Xuzhou:China University of Mining and Technology Press, 2010. [25] 刘艳华, 车得福, 李荫堂, 惠世恩, 徐通模. X-射线光电子能谱确定铜川煤及其焦中氮的形态[J].西安交通大学学报, 2001, 35(7):661-665. http://www.cnki.com.cn/Article/CJFDTOTAL-XAJT200107000.htmLIU Yan-hua, CHE De-fu, LI Yin-tang, HUI Shi-en, XU Tong-mo. X-ray photoelectron spectroscopy determination of the forms of nitrogen in Tongchuan coal and its chars[J]. J Xi'an Jiaotong Univ, 2001, 35(7):661-665. http://www.cnki.com.cn/Article/CJFDTOTAL-XAJT200107000.htm [26] 吴婷, 凌凤香, 马波, 王少军, 吴洪新. GC-MS分析低温煤焦油酸性组分及碱性组分[J].石油化工高等学校学报, 2013, 26(3):44-52. http://www.cnki.com.cn/Article/CJFDTOTAL-SYHX201303009.htmWU Ting, LING Feng-xiang, MA Bo, WANG Shao-jun, WU Hong-xin. Analysis of acidcomposition and base composition from low-temperature coal tar by GC-MS[J]. J Petrochem Univ, 2013, 26(3):44-52. http://www.cnki.com.cn/Article/CJFDTOTAL-SYHX201303009.htm [27] 张喜亮, 贾德民. 炭黑表面的化学结构模型[C]. 全国高分子学术论文报告会, 2001.ZHANG Xi-liang, JIA De-min. Chemical model of carbon black surface[C]. Nation Polymer Academic Paper Report, 2001. [28] 王道宏, 王日杰, 张继炎, 何菲. X光电子能谱法研究色素碳黑的表面化学性质[J].天津大学学报, 2004, 37(7):634-638. http://www.cnki.com.cn/Article/CJFDTOTAL-TJDX200407015.htmWANG Dao-hong, WANG Ri-jie, ZHANG Ji-yan, HE Fei. Study on surface chemical property of carbon black pigments by x-ray photoelectronic spectroscopy[J]. J Tianjin Univ, 2004, 37(7):634-638. http://www.cnki.com.cn/Article/CJFDTOTAL-TJDX200407015.htm [29] 朱廷钰, 汤忠, 黄戒介, 张建民, 汪洋.煤温和气化特性的热重研究[J].燃料化学学报, 1999, 27(5):420-423. http://www.cnki.com.cn/Article/CJFDTOTAL-RLHX199905008.htmZHU Ting-yu, TANG Zhong, HUANG Jie-jie, ZHANG Jian-min, WANG Yang. Thermo-gravimetric study of coal mild gasification[J]. J Fuel Chem Technol, 1999, 27(5):420-423. http://www.cnki.com.cn/Article/CJFDTOTAL-RLHX199905008.htm [30] TIAN F J, YU J L, MCKENZIE L J, HAYASHI J I, LI C Z. Formation of NOx precursors during the pyrolysis of coal and biomass. Part IX. Effect of coal ash and externally loaded-Na on fuel-N conversion during the reforming of coal and biomass in steam[J]. Fuel, 2006, 85(10/11):1411-1417. [31] LI C Z, LI L T. Formation of NOx and SOx precursors during the pyrolysis of coal and biomass. Part III. Further discussion on the formation of HCN and NH3 during pyrolysis[J]. Fuel, 2000, 79(15):1899-1906. doi: 10.1016/S0016-2361(00)00008-9 [32] 熊杰, 周志杰, 许慎启, 于广锁.碱金属对煤热解和气化反应速率的影响[J].化工学报, 2011, 62(1):192-198. http://www.cnki.com.cn/Article/CJFDTOTAL-HGSZ201101031.htmXIONG JIE, ZHOU Zhi-jie, XU Shen-qi, YU Guang-suo. Effect of alkali metal on rate of coal pyrolysis and gasification[J]. CIESC J, 2011, 62(1):192-198. http://www.cnki.com.cn/Article/CJFDTOTAL-HGSZ201101031.htm [33] KAPTEIGIN F, MOULIGIN J A, MATZNER S, BOEHMB H P. The development of nitrogen functionality in model chars during gasification in CO2 and O2[J]. Carbon, 1999, 37(7):1143-1150. doi: 10.1016/S0008-6223(98)00312-1 [34] SCHMIER S H, FRIEBEL J, STREUBEL P, HESSEB R K, PSELA R. Change of chemical bonding of nitrogen of polymeric N-heterocyclic compounds during pyrolysis[J]. Carbon, 1999, 37(12):1965-1978. doi: 10.1016/S0008-6223(99)00071-8 [35] XU M X, LI S Y, WU Y H, JIA L F, LU Q G. Effects of CO2 on the fuel nitrogen conversion during coal rapid pyrolysis[J]. Fuel, 2016, 184:430-439. doi: 10.1016/j.fuel.2016.06.130 -





 下载:
下载: