Experimental study on selective catalytic reduction of NO with propene over iron based catalysts supported on aluminum pillared clays
-
摘要: 采用浸渍法制备了负载于铝柱撑黏土的铁基催化剂(Fe/Al-PILC),在固定床反应器上测试其催化C3H6选择性还原NO的性能。通过N2吸附-脱附、X射线衍射(XRD)、H2的程序升温还原(H2-TPR)、紫外可见光谱(Uv-vis)、吡啶吸附红外光谱(Py-FTIR)等手段对催化剂的物理化学性质进行表征。结果表明,9Fe/Al-PILC在400-550 ℃能够还原98%以上的NO,而且SO2和水蒸气对其催化性能的影响很小。XRD、N2吸附-脱附表征结果表明,Fe/Al-PILC催化剂中铁氧化物高度分散在载体表面,催化剂有较大的比表面积和孔容。H2-TPR结果表明,催化剂的活性主要由Fe2O3物相的还原性能决定。Uv-vis结果表明,催化剂的活性与铁氧低聚物种FexOy呈正相关性。Py-FTIR结果表明,催化剂表面同时存在Lewis酸和Brønsted酸,L酸性位是NO和C3H6反应的主要催化活性中心。Abstract: Iron based catalysts supported on aluminum pillared clays (Fe/Al-PILC) was prepared by impregnation method and the selective catalytic reduction of NO with propene by the Fe/Al-PILC catalysts was investigated in a fixed bed reactor. The physical and chemical properties of the catalysts were characterized by N2 adsorption/desorption, XRD, H2-TPR, Uv-vis, Py-FTIR, etc. Results showed that 9% Fe/Al-PILC reduced more than 98% of NO at 400-550℃. SO2 and water vapor slightly influenced the catalytic activity of the catalysts. XRD and N2 adsorption/desorption characterization results revealed that the iron oxides in the catalyst were highly dispersed on the surface of the carrier, and the catalyst has a large specific surface area and pore volume. H2-TPR results indicated that the activity of the catalysts was mainly determined by the reduction performance of Fe2O3 phase. Uv-vis results showed that the activity of the catalysts was positively correlated with the iron oxide oligomer FexOy. Py-FTIR results indicated that both Lewis acid and Brønsted acid were formed on the catalyst surface and the Lewis acid sites were the main catalytic activity center of NO and C3H6 reaction.
-
Key words:
- NO /
- selective catalytic reduction /
- Al-PILC /
- iron /
- C3H6
-
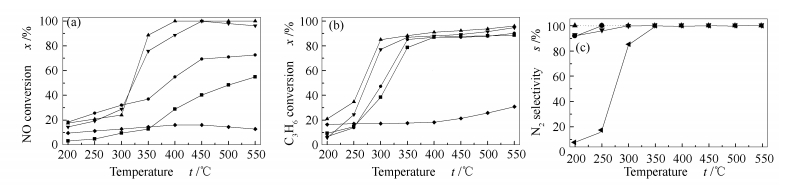
图 1 不同催化剂取得的NO和C3H6转化率及N2选择性随反应温度的变化
Figure 1 NO conversion to N2(a), C3H6 conversion (b), N2 selectivity(c) over Fe/Al-PILC with different iron loading
reaction conditions: φNO=0.05%, φC3H6=0.3%, φO2 =1%, He=balance and GHSV=15 000 h-1
—■—4Fe/Al-PILC; —●—6Fe/Al-PILC; —▲—9Fe/Al-PILC; —▼—13Fe/Al-PILC; —◆—Al-PILC表 1 不同催化剂的物理特性
Table 1 Physical property of different catalysts
Catalyst Fe w/ (mg·g-1) ABET /(m2·g-1) Pore volume v/(cm3·g-1) Pore diameter d/nm Al-PILC 188 0.328 5.61 4Fe/Al-PILC 40.19 164 0.258 5.52 9Fe/Al-PILC 92.54 186 0.264 4.91 13Fe/Al-PILC 131.19 65 0.097 4.84 表 2 不同催化剂的B酸和L酸含量
Table 2 Brønsted and Lewis acid content of different catalysts
Sample 170 ℃ desorption /(mmol·g-1) 300 ℃ desorption /(mmol·g-1) B L B L Al-PILC 0.005 12 0.051 29 0 0.035 80 4Fe/Al-PILC 0.003 97 0.025 80 0 0.011 40 9Fe/Al-PILC 0.006 91 0.046 95 0 0.034 43 -
[1] IWAMOTO M, YAHIRO H, YU U Y.Selective reduction of NO by lower hydrocarbons in the presence of O2 and SO2 over copper ion-exchanged zeolites[J]. Catal, 1990, 32(6):430-433. [2] HELD W, KOENIG A, RICHTER T, PUPPE L. Catalytic NOx reduction in net oxidizing exhaust gas[J]. SAE Trans, 1990, 99(4):209-216. http://www.researchgate.net/publication/285442659_Catalytic_NOx_reduction_in_net_oxidizing_exhaust_gas [3] 周皞, 苏亚欣, 邓文义, 钟方川.金属氧化物类催化剂上HC-SCR研究进展[J].环境科学与技术, 2016, 39(1):93-100. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=fjks201601015&dbname=CJFD&dbcode=CJFQZHOU Hao, SU Ya-xin, DENG Wen-yi, ZHONG Fang-chuan. A Review of HC-SCR over Metal Oxides-based Catalysts[J].Environ Sci Technol, 2016, 39(1):93-100. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=fjks201601015&dbname=CJFD&dbcode=CJFQ [4] 周皞, 苏亚欣, 邓文义, 钟方川.负载金属分子筛类催化剂上HC-SCR研究进展[J].环境科学与技术, 2015, 38(10):64-73. http://www.cqvip.com/QK/90776X/201510/666977573.htmlZHOU Hao, SU Ya-xin, DENG Wen-yi, ZHONG Fang-chuan. A Review of HC-SCR over Metal-based Zeolite Catalysts[J].Environ Sci Technol, 2015, 38(10):64-73. http://www.cqvip.com/QK/90776X/201510/666977573.html [5] YASHNIK S A, SALNIKOV A V, VASENIN N T, ANUFRIENKO V F, ISMAGILOV Z R. Regulation of the copper-oxide cluster structure and DeNOx activity of Cu-ZSM-5 catalysts by variation of OH/Cu2+[J]. Catal Today, 2012, 197(1):214-227. doi: 10.1016/j.cattod.2012.08.033 [6] KUMAR P A, REDDY M P, JU LK, HYUN-SOOK B, PHIL H H. Low temperature propylene SCR of NO by copper alumina catalyst[J]. J Mol Catal A Chem, 2008, 291(1/2):66-74. http://www.sciencedirect.com/science/article/pii/S1381116908002239 [7] LONG R Q, CHANG M T, YANG R T. Enhancement of activities by sulfation on Fe-exchanged TiO2-pillared clay for selective catalytic reduction of NO by ammonia[J]. Appl Catal B:Environ, 2001, 33(2):97-107. doi: 10.1016/S0926-3373(01)00173-4 [8] VAUGHAN D E W, LUSSIER R J, MAGEE J S. Pillared interlayered clay materials useful as catalysts and sorbents, CA, 1979. http://www.freepatentsonline.com/4176090.html [9] LIN Q C, HAO J M, LI J H, MA Z F, LIN W M. Copper-impregnated Al-Ce-pillared clay for selective catalytic reduction of NO by C3H6[J]. Catal Today, 2007, 126(3/4):351-358. http://www.sciencedirect.com/science/article/pii/S0920586107003525 [10] DORADO F, LUCAS A D, GARCÍA P B, VALVERDE J L, ROMERO A. Preparation of Cu-ion-exchanged Fe-PILCs for the SCR of NO by propene[J]. Appl Catal B:Environ, 2006, 65(3/4):175-184. http://www.sciencedirect.com/science/article/pii/S0926337306000294 [11] YANG R T, THARAPPIWATTANANON N, LONG R Q. Ion-exchanged pillared clays for selective catalytic reduction of NO by ethylene in the presence of oxygen[J]. Appl Catal B:Environ, 1998, 19(3/4):289-304. http://www.sciencedirect.com/science/article/pii/S0926337398000836 [12] LU G, LI X Y, QU Z P, ZHAO Q D, ZHAO L, CHEN G H. Copper-ion exchanged Ti-pillared clays for selective catalytic reduction of NO by propylene[J]. Chem Eng J, 2011, 168(3):1128-1133. doi: 10.1016/j.cej.2011.01.095 [13] 苏亚欣, 任立铭, 苏阿龙, 邓文义.甲烷在金属铁及氧化铁表面还原NO的研究[J].燃料化学学报, 2013, 41(11):1393-1400. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18303.shtmlSU Ya-xin, REN Li-ming, SU A-long, DENG Wen-yi. Experimental study on NO reduction by methane over iron and its oxides[J]J Fuel Chem Technol, 2013, 41(11):1393-1400. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18303.shtml [14] 苏亚欣, 陆哲惺, 周皞, 窦逸峰, 邓文义.丙烷在金属铁表面还原NO的实验研究[J].燃料化学学报, 2014, 42(12):1470-1477. doi: 10.3969/j.issn.0253-2409.2014.12.009SU Ya-xin, LU Zhe-xing, ZHOU Hao, DOU Yi-feng, DENG Wen-yi.Experimental study on NO reduction by propane over iron[J].J Fuel Chem Technol, 2014, 42(12):1470-1477. doi: 10.3969/j.issn.0253-2409.2014.12.009 [15] 窦逸峰, 苏亚欣, 陆哲惺, 周皞, 邓文义.乙烷在金属铁表面还原NO的实验研究[J].燃料化学学报, 2015, 43(10):1273-1280. doi: 10.3969/j.issn.0253-2409.2015.10.017DOU Yi-feng, SU Ya-xin, LU Zhe-xing, ZHOU Hao, DENG Wen-yi.Experimental study of NO reduction by ethane over iron[J].J Fuel Chem Technol, 2015, 43(10):1273-1280. doi: 10.3969/j.issn.0253-2409.2015.10.017 [16] 梁俊青, 苏亚欣, 周皞, 邓文义.金属铁与丙烯共同还原NO的特性与机理[J].燃料化学学报, 2016, 44(8):977-984. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18883.shtmlLIANG Jun-qing, SU Ya-xin, ZHOU Hao, DENG Wen-yi. Performance and mechanism of NO reduction by iron combined with propene[J]. J Fuel Chem Technol, 2016, 44(8):977-984. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18883.shtml [17] 苏亚欣, 苏阿龙, 任立铭, 邓文义. SO2对甲烷在金属铁表面还原NO的反应影响[J].燃料化学学报, 2014, 42(3):377-384. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18382.shtmlSU Ya-xin, SU A-long, REN Li-ming, DENG Wen-yi. Effect of SO2 on NO reduction by methane over iron[J].J Fuel Chem Technol, 2014, 42(3):377-384. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18382.shtml [18] 周皞, 苏亚欣, 戚越舟, 陆哲惺, 邓文义.水蒸气对甲烷在金属铁表面还原NO行为的影响[J].燃料化学学报, 2014, 42(11):1378-1386. doi: 10.3969/j.issn.0253-2409.2014.11.016ZHOU Hao, SU Ya-xin, QI Yue-zhou, LU Zhe-xing, DENG Wen-yi.Effect of water vapor on NO reduction by methane over iron[J].J Fuel Chem Technol, 2014, 42(11):1378-1386. doi: 10.3969/j.issn.0253-2409.2014.11.016 [19] ERKFELDT S, PALMQVIST A, PETERSSON M. Influence of the reducing agent for lean NOx reduction over Cu-ZSM-5[J]. Appl Catal B:Environ, 2011, 102(3/4):547-554. http://www.sciencedirect.com/science/article/pii/S0926337310005758 [20] YANG T T, BI H T, CHENG X X. Effects of O2, CO2 and H2O on NOx adsorption and selective catalytic reduction over Fe/ZSM-5[J]. Appl Catal B:Environ, 2011, 102(1/2):163-171. http://www.sciencedirect.com/science/article/pii/S0926337310005266 [21] CHEN S W, YAN X L, WANG Y, CHEN S W, YAN X L, WANG Y, CHEN J Q, PAN D H, MA J H, LI R F. Effect of SO on Co sites for NO-SCR by CH over Co-Beta[J]. Catal Today, 2011, 175:12-17. doi: 10.1016/j.cattod.2011.05.024 [22] LÓNYI F, SOLT H E, VALYON J, BOIX A, GUTIERREZ L B. The SCR of NO with methane over In, H-and Co, In, H-ZSM-5 catalysts:The promotional effect of cobalt[J]. Appl Catal B:Environ, 2012, s117/118(1):212-223. http://www.sciencedirect.com/science/article/pii/S0926337312000239 [23] LÓNYI F, SOLT H E, PÁSZTI Z, VALYON J. Mechanism of NO-SCR by methane over Co, H-ZSM-5 and Co, H-mordenite catalysts[J]. Appl Catal B:Environ, 2014, s150/151(18):218-229. http://www.sciencedirect.com/science/article/pii/S0926337313007698 [24] GARCÍA P B, DORADO F, LUCAS A D, LUCAS-CONSUEGRA A D, NIETO-MÁRQUEZ A, VALVERDE J L, ROMERO A. SCR of NO by C3H6 over Cu-Fe-PILC in the presence of oxygen and steam[J]. Proceedings of European Congress of Chemical Engineering (ECCE-6). Copenhagen, 16-20 September 2007. [25] ZHOU H, SU Y X, LIAO W Y, DENG W Y, ZHONG F C. NO reduction by propane over monolithic cordierite-based Fe/Al2O3 catalyst:Reaction mechanism and effect of H2O/SO2[J]. Fuel, 2016, 182:352-360. doi: 10.1016/j.fuel.2016.05.116 [26] LONG R Q, YANG R T. Selective catalytic reduction of nitrogen oxides by ammonia over Fe3+-exchanged TiO2 -pillared cay catalysts[J]. J Catal, 1999, 186(2):254-268. doi: 10.1006/jcat.1999.2558 [27] HUANG Q Q, ZUO S F, ZHOU R X. Catalytic performance of pillared interlayered clays (PILCs) supported CrCe catalysts for deep oxidation of nitrogen-containing VOCs[J]. Appl Catal B:Environ, 2010, 95(3):327-334. http://www.sciencedirect.com/science/article/pii/S092633731000024X [28] DORADO F, GARCÍA P B, LUCAS A D, RAMOS M J, ROMERO A. Hydrocarbon selective catalytic reduction of NO over Cu/Fe-pillared clays:Diffuse reflectance infrared spectroscopy studies[J].J Mol Catal A:Chem 2010, 332(1/2):45-52. http://www.sciencedirect.com/science/article/pii/S1381116910003596 [29] CHMIELARZ L, PIWOWARSKA Z, KU'STROWSKI P, WEGRZYN A, GIL B, KOWALCZYK A, DUDEK B, DZIEMBAJ R, MICHALIK M. Comparison study of titania pillared interlayered clays and porous clay heterostructures modified with copper and iron as catalysts of the DeNOx process[J]. Appl Clay Sci, 2011, 53(2):164-173. doi: 10.1016/j.clay.2010.12.009 [30] 沈伯雄, 马宏卿, 杨晓燕, 姚燕. Mn-CeOx/Ti-PILC的制备、表征及脱硝性能研究[J].燃料化学学报, 2012, 40(5):615-620. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract17952.shtmlSHEN Bo-xiong, MA Hong-qing, YANG Xiao-yan, YAO Yan. Study on preparation, characterization and de-NO activity of Mn-CeOx/Ti-PILC[J]. J Fuel Chem Technol. 2012, 40(5):615-620. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract17952.shtml [31] ZHOU H, SU Y X, LIAO W Y, DENG W Y, ZHONG F C. Preparation, characterization, and properties of monolithic Fe/Al2O3/cordierite catalysts for NO reduction with C2H6[J]. Appl Catal A:Gen, 2015, 505:402-409. doi: 10.1016/j.apcata.2015.08.025 [32] 叶青, 闫立娜, 霍飞飞, 王海平, 程水源, 康天放. Fe柱撑海泡石负载Cu催化剂:结构特点及其C3H6选择性催化还原NO催化性质[J].无机化学学报, 2012, 28(1):103-112. http://d.wanfangdata.com.cn/Periodical/wjhxxb201201018YE Qing, YAN Li-Na, HUO Fei-Fei, WANG Hai-Ping, CHENG Shui-Yuan KANG Tian-Fang. Cu-Supportedon Fe-Pillared Sepiolite:Characterization and Selective Catalytic Reduction(SCR)of NO by Propene[J]. Chin J Inorg Chem, 2012, 28(1):103-112. http://d.wanfangdata.com.cn/Periodical/wjhxxb201201018 [33] OLVEIRA L C A, RIOS R V R A, FABRIS J D, SAPAG K, GARG V K, LAGO R M. Clay-iron oxide magnetic composites for the adsorption of contaminants in water[J]. Appl Clay Sci, 2003, 22(4):169-177. doi: 10.1016/S0169-1317(02)00156-4 [34] BRANDENBERGER S, KRÖCHER O, WOKAUN A, TISSLER A, ALTHOFF R. The role of Brønsted acidity in the selective catalytic reduction of NO with ammonia over Fe-ZSM-5[J]. J Catal, 2009, 268(2):297-306. doi: 10.1016/j.jcat.2009.09.028 [35] FIERRO G, MORETTI G, Ferraris G, ANDREOZZI G B. A Mössbauer and structural investigation of Fe-ZSM-5 catalysts:Influence of Fe oxide nanoparticles size on the catalytic behaviour for the NO-SCR by C3H8[J]. Appl Catal B:Environ, 2011, 102(1/2):215-223. http://www.sciencedirect.com/science/article/pii/S0926337310005321 [36] DATKA J, TUREK A M, JEHNG J M, WACHS I E. Acidic properties of supported niobium oxide catalysts:An infrared spectroscopy investigation[J]. J Catal, 1992, 135(135):186-199. http://www.sciencedirect.com/science/article/pii/002195179290279Q [37] SULTANA A, HANEDA M, FUJITANI T, HAMADA H. Influence of Al2O3 support on the activity of Ag/Al2O3 catalysts for SCR of NO with decane[J]. Catal Lett, 2007, 114(1):96-102. doi: 10.1007/s10562-007-9045-5 [38] PERDIGON-MELON J A, GERVASINI A, AUROUX A. Study of the influence of the In2O3 loading on γ-alumina for the development of de-NOx catalysts[J]. J Catal, 2005, 234(2):421-430. doi: 10.1016/j.jcat.2005.07.001 -





 下载:
下载: