Effect of ethylene glycol on the hydrogenation performance of P-doped NiMo/Al2O3 catalysts
-
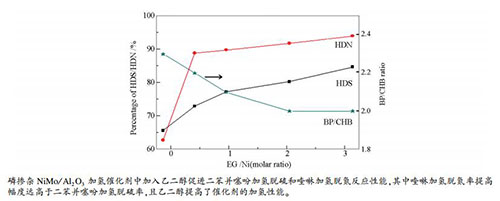
摘要: 采用NiMoP浸渍液中添加乙二醇(EG)的方式制备了不同EG含量的NiMoP(x)/Al2O3催化剂,为研究EG及其含量对该系列催化剂催化性能和活性相结构的影响,用二苯并噻吩(DBT)和喹啉(Q)为模型化合物,考察了催化剂的加氢脱硫(HDS)和加氢脱氮(HDN)性能。结果表明,在EG添加量较低的情况下(EG/Ni物质的量比分别为0、0.5、1、2、3),EG能够明显提高催化剂对DBT和Q的HDS和HDN活性,其中,HDN活性提高幅度大于HDS,且随着EG含量提高,催化剂的HDS和HDN活性进一步提高。通过TEM分析和XPS分析可知,EG有助于增加催化剂中MoS2颗粒的堆积层数和片层长度,且随着EG含量增加,堆积层数和片层长度都有所增加;EG有助于提高Mo表面原子浓度,对Ni表面原子浓度影响较小,但明显提高了Mo和Ni硫化程度。TG表征说明,EG在氧化铝和催化剂表面存在多种相互作用方式,并且存在与活性组分相互作用的耐高温有机物种。Abstract: NiMoP(x)/Al2O3 catalysts with different ethylene glycol (EG) contents were prepared by impregnating NiMoP solution containing EG into Al2O3. The hydrodesulfurization (HDS) and hydrodenitrogenation (HDN) performances of the catalysts were evaluated using dibenzothiophene (DBT) and quinoline (Q) as the model compounds. The results showed that the HDS and HDN performances of the catalysts could be improved by adding of EG when the amount of EG was low (nEG/nNi ratio value was 0, 0.5, 1, 2, 3, respectively), and the improvement of HDN performance was more obvious than HDS performance. With the increase of EG content, the activity of HDS and HDN of the catalysts was further improved. TEM and XPS analysis showed that EG was helpful to increase the stacking layer number and lamellar length of MoS2 particles in the catalysts, and with the increase of EG content, the stacking layer number and lamellar length of MoS2 particles increased. EG could improve the surface atomic concentration of Mo, but practically had no influence on the surface atomic distribution of Ni. However, EG significantly increased the sulfuration degree of Mo and Ni. TG characterization showed that EG interacted with alumina and metal active components in various ways, and there were high temperature resistant organic species interacting with active components.
-
Key words:
- ethylene glycol /
- catalyst /
- hydrodesulfurization /
- hydrodenitrogenation
-
表 1 DBT在NiMoP(x)/Al2O3催化剂上HDS产物分布
Table 1 Product distribution of DBT HDS over the NiMoP(x)/Al2O3 catalysts
Catalyst wmol/% NiMoP(0)/Al2O3 NiMoP(0.5)/Al2O3 NiMoP(1)/Al2O3 NiMoP(2)/Al2O3 NiMoP(3)/Al2O3 
0.14 0.33 0.14 0.15 0.13 
0.64 0.77 0.68 0.69 0.66 
0.00 0.00 0.00 0.00 0.00 
19.91 22.76 24.61 26.61 27.52 
44.87 51.28 51.76 52.83 56.38 
0.21 0.66 0.14 0.15 0.10 
0.43 0.63 0.59 0.35 0.27 
33.8 23.57 22.07 19.21 14.88 表 2 Q在NiMoP(x)/Al2O3催化剂HDN产物分布
Table 2 Product distribution of quinoline HDN over the NiMoP(x)/Al2O3 catalysts
Catalyst wmol/% NiMoP(0)/Al2O3 NiMoP(0.5)/Al2O3 NiMoP(1)/Al2O3 NiMoP(2)/Al2O3 NiMoP(3)/Al2O3 
54.21 74.63 84.31 86.87 88.88 
7.68 5.86 5.52 5.23 5.07 
0.79 0 0 0 0 
1.13 0 0 0 0 
0 0 0 0 0 
1.45 0.18 0.15 0.17 0.13 
29.31 18.64 9.72 7.48 5.72 
0 0 0 0 0 
5.42 1.06 0.30 0.81 0.20 表 3 MoS2颗粒的平均堆垛层数(NA)、平均片长(LA)以及可暴露Mo原子比(fMo)
Table 3 Average stacking number (NA), average slab length (LA) and fraction of available Mo (fMo) of MoS2
Catalyst NA LA /nm fMo NiMoP(0)/Al2O3 1.6 3.95 0.267 NiMoP(0.5)/Al2O3 1.7 4.30 0.247 NiMoP(2)/Al2O3 2.0 4.32 0.252 NiMoP(3)/Al2O3 2.5 4.60 0.222 表 4 反应后催化剂Mo 3d和Ni 2p的结合能
Table 4 Mo 3d and Ni 2p binding energy of the spent catalysts
Catalyst Binding energy EB/eV Mo 3d5/2 Mo 3d3/2 Ni 2p3/2 NiMoP(0)/Al2O3 229.3, 231.5, 233.5 232.5, 234.7, 236.5 852.9, 854.2, 856.3 NiMoP(0.5)/Al2O3 229.1, 231.4, 232.9 232.3, 234.6, 236.1 853.3, 853.9, 856.1 NiMoP(2)/Al2O3 229.1, 231.6, 233.1 232.3, 234.8, 236.3 853.3, 854.1, 855.9 NiMoP(3)/Al2O3 229.2, 231.6, 233.2 232.4, 234.8, 236.4 853.3, 854.2, 856.3 表 5 反应后催化剂表面Ni、Mo物质的量比
Table 5 Atomic ratios of Ni, Mo of the spent catalysts
Parameter NiMoP(0)/Al2O3 NiMoP(0.5)/Al2O3 NiMoP(2)/Al2O3 NiMoP(3)/Al2O3 Mo(3d) MoS2/Al 0.115 0.128 0.135 0.139 Mo5+/Al 0.015 0.016 0.017 0.018 Mo6+/Al 0.021 0.021 0.020 0.021 Mo4+/Mototal 0.762 0.776 0.785 0.781 Mototal/Al 0.151 0.165 0.172 0.178 Ni (2p) Ni-sulf/Al 0.017 0.019 0.023 0.022 NiMoS/Al 0.030 0.031 0.028 0.038 Ni2+/Al 0.024 0.012 0.009 0.008 Ni(总)/Al 0.064 0.062 0.060 0.068 -
[1] GAO Y, HAN W, LONG X Y. Preparation of hydrodesulfurization catalysts using MoS3 nanoparticles as a precursor[J]. Appl Catal B:Environ, 2018, 224:330-340. doi: 10.1016/j.apcatb.2017.10.046 [2] PRAJAPATI Y N, VERMA N. Hydrodesulfurization of thiophene on activated carbon fiber supported NiMo catalysts[J]. Energy Fuels, 2018, 32(2):2183-2196. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=6bbb5e245c119d94792394f811f2d990 [3] 徐艳春, 李翔, 王安杰, 遇治权, 陈永英.镧氧化物对体相MoP加氢脱氮反应性能的影响[J].石油学报(石油加工), 2018, 34(1):115-122. doi: 10.3969/j.issn.1001-8719.2018.01.016XU Yan-chun, LI Xiang, WANG An-jie, YU Zhi-quan, CHEN Yong-ying. Influence of lanthanum oxide on the hydrodenitrogenation performance of bulk MoP[J]. Acta Pet Sin (Pet Process Sect), 2018, 34(1):115-122. doi: 10.3969/j.issn.1001-8719.2018.01.016 [4] NICOSIA D, PRINS R. The effect of glycol on phosphate-doped CoMo/Al2O3 hydrotreating catalysts[J]. J Catal, 2005, 229(2):424-438. doi: 10.1016/j.jcat.2004.11.014 [5] VAN HAANDEL L, BREMMER G M, HENSEN E J M, WEBER T. The effect of organic additives and phosphoric acid on sulfiation and activity of (Co)Mo/Al2O3 hydrodesulfurization catalysts[J]. J Catal, 2017, 351:95-106. doi: 10.1016/j.jcat.2017.04.012 [6] NICOSIA D, PRINS R. The effect of phosphate and glycol on the sulfidation mechanism of CoMo/Al2O3 hydrotreating catalysts:An in situ QEXAFS study[J]. J Catal, 2005, 231(2):259-268. doi: 10.1016/j.jcat.2005.01.018 [7] STANISLAUS A, MARAFI A, RANA M S. Recent advances in the science and technology of ultra-low sulfur diesel (ULSD) production[J]. Catal Today, 2010, 153(1/2):1-68. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=c3a5d46f7b195464cfb15c24c522231a [8] ITO E, VAN VEEN J A R. On novel processes for removing sulphur from refinery streams[J]. Catal Today, 2006, 116(4):446-460. doi: 10.1016/j.cattod.2006.06.040 [9] PIMERZIN A, MOZHAEV A, VARAKIN A, MASLAKOV K, NIKULSHIN P. Comparison of citric acid and glycol effects on the state of active phase species and catalytic properties of CoPMo/Al2O3 hydrotreating catalysts[J]. Appl Catal B:Environ, 2017, 205:93-103. doi: 10.1016/j.apcatb.2016.12.022 [10] ESCOBAR J, BARRERA M C, TOLEDO J A, CORTES-JACOME M A, ANGELES-CHAVEZ C, NUNEZ S, SANTES V, GOMEZ E, DIAZ L, ROMERO E, PACHECO J G. Effect of ethyleneglycol addition on the properties of P-doped NiMo/Al2O3 HDS catalysts:Part Ⅰ. Materials preparation and characterization[J]. Appl Catal B:Environ, 2009, 88(3/4):564-575. https://www.sciencedirect.com/science/article/abs/pii/S0926337308003871 [11] IWAMOTO R, KAGAMI N, SAKODA Y, IINO A. Effect of polyethylene glycol addition on NiO-MoO3/Al2O3 and NiO-MoO3-P2O5/Al2O3 hydrodesulfurization catalyst[J]. J Jpn Pet Inst, 2005, 48(6):351-357. doi: 10.1627/jpi.48.351 [12] 韩文鹏, 张晔, 李学宽, 唐兴明, 周立公, 吴明红, 葛晖.配合物的配位基团性质对CoMo/Al2O3催化剂加氢脱硫性能的影响[J], 燃料化学学报, 2017, 45(11):1332-1339. doi: 10.3969/j.issn.0253-2409.2017.11.008HAN Wen-peng, ZHANG Ye, LI Xue-kuan, TANG Xing-ming, ZHOU Li-gong, WU Ming-hong, GE Hui. Effect of coordinating groups of chelating agents on the hydrodesulfurization over CoMo/Al2O3 catalysts[J]. J Fuel Chem Technol, 2017, 45(11):1332-1339. doi: 10.3969/j.issn.0253-2409.2017.11.008 [13] ROB VAN VEEN J A. What's new? On the development of sulphidic HT catalysts before the molecular aspects[J]. Catal Today, 2017, 292:2-25. doi: 10.1016/j.cattod.2016.09.027 [14] COSTA V, GUICHARD B, DIGNE M, LEGENS C, LECOUR P, MARCHAND K, RAYBAUD P, KREBS E, GEANTET C. A rational interpretation of improved catalytic performances of additive-impregnated dried CoMo hydrotreating catalysts:A combined theoretical and experimental study[J].Catal Sci Technol, 2013, 3(1):140-151. doi: 10.1039/C2CY20553J [15] NGUYEN T S, LORIDANT S, CHANTAL L, CHOLLEY T, GEANTET C. Effect of glycol on the formation of active species and sulfidation mechanism of CoMoP/Al2O3 hydrotreating catalysts[J]. Appl Catal B:Environ, 2011, 107(1/2):59-67. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=6d590f9db787fbdbe0355b468b62466b [16] IWAMOTO R, KAGAMI N, IINO A. Effect of polyethylene glycol addition on hydrodesulfurization activity over CoO-MoO3/Al2O3 catalyst[J]. J Jpn Pet Inst, 2005, 48(4):237-242. doi: 10.1627/jpi.48.237 [17] GUTIERREZ-ALEJANDRE A, LAURRABAQUIO-ROSAS G, RAMIREZ J, BUSCA G. On the role of triethyleneglycol in the preparation of highly active Ni-Mo/Al2O3 hydrodesulfurization catalysts:A spectroscopic study[J]. Appl Catal B:Environ, 2015, 166/167:560-567. doi: 10.1016/j.apcatb.2014.11.039 [18] ALEXEY L N, GALINA A B, ALEKSANDER A P, IGOR P P, IRINA V D, VLADIMIR A V, EVGENY Y G, EVGENIYA N V, VALERII I B. Effect of Mono-, Di-, and triethylene glycol on the activity of phosphate-doped NiMo/Al2O3 hydrotreating catalysts[J], Catalysts, 2019, 9(1):96. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=MDPI000000211687 [19] 罗锡辉, 何金海.一种催化剂浸渍液及其配制方法: 中国, 961090480[P]. 2000-11-01.LUO Xi-hui, HE Jin-hai. A Catalyst Impregnate and Its Preparation Method: CN, 961090480[P]. 2000-11-01. [20] FERDOUS D, DALAI A K, ADJAYE J. A series of NiMo/Al2O3 catalysts containing boron and phosphorous Part Ⅰ. Synthesis and characterization[J]. Appl Catal A:Gen, 2004, 260(1):137-151. [21] QU L L, ZHANG W P, KOOYMAN P J, PRINS R. MAS NMR, TPR, and TEM studies of the interaction of NiMo with alumina and silica-alumina supports[J]. J Catal, 2003, 215(1):7-13. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=dedb322a50e2e953e01c0aa77837d640 [22] SOLIS D, AGUDO A L, RAMIREZ J, KLIMOVA T. Hydrodesulfurization of hindered dibenzothiophenes on bifunctional NiMo catalysts supported on zeolite-alumina composites[J]. Catal Today, 2006, 116(4):469-477. doi: 10.1016-j.cattod.2006.06.029/ [23] SUN M Y, KOOYMAN P J, PRINS R. A high-resolution transmission electron microscopy study of the influence of fluorine on the morphology and dispersion of WS2 in sulfided W/Al2O3 and NiW/Al2O3 catalysts[J]. J Catal, 2002, 206(2):368-375. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=6c7bcdad264633773572cb42d8ee8b74 [24] HENSEN E J M, KOOYMAN P J, VAN DER MEER Y, VAN DER KRAAN A M, DE BEER V H J, VAN VEEN J A R, VAN SANTEN R A. The relation between morphology and hydrotreating activity for supported MoS2 particles[J]. J Catal, 2001, 199(2):224-235. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=c2c14964a54421c417312989dcacbea9 [25] VRADMAN L, LANDAU M V, HERSKOWITZ M. Hydrodearomatization of petroleum fuel fractions on silica supported Ni-W sulphide with increased stacking number of the WS2 phase[J]. Fuel, 2003, 82(6):633-639. doi: 10.1016/S0016-2361(02)00354-X [26] FARAG H, SAKANISHI K, KOUZU M, MATSUMURA A, SUGIMOTO Y, SALTO I. Dibenzothiophene hydrodesulfurization over synthesized MoS2 catalysts[J]. J Mol Catal A:Chem, 2003, 206(1/2):399-408. http://d.old.wanfangdata.com.cn/NSTLQK/NSTL_QKJJ0210429839/ [27] SHIDO T, PRINS R. Why EXAFS underestimated the size of small supported MoS2 particles[J]. J Phys Chem B, 1998, 102(43):8426-8435. doi: 10.1021/jp982322j [28] CALAIS C, MATSUBAYASHI N, GEANTET C, YOSHIMURA Y, SHIMADA H, NISHIJIMA A, LACROIX M, BREYSSE M. Crystallite size determination of highly dispersed unsupported MoS2 catalysts[J]. J Catal, 1998, 174(2):130-141. doi: 10.1006/jcat.1998.1934 [29] DE LA ROSA M P, TEXIER S, BERHAULT G, CAMACHO A, YACAMAN M J, MEHTA A, FUENTES S, MONTOYA J A, MURRIETA F, CHIANELLI R R. Structural studies of catalytically stabilized model and industrial-supported hydrodesulfurization catalysts[J]. J Catal, 2004, 225(2):288-299. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=37994817dbbe86d7a7ec9d34fb36a423 [30] CHIANELLI R R. Periodic trends transition metal sulfide catalysis:Intuition and theory[J]. Oil Gas Sci Technol, 2006, 61(4):503-513. doi: 10.2516/ogst:2006022a [31] EIJSBOUTS S. On the flexibility of the active phase in hydrotreating catalysts[J]. Appl Catal A:Gen, 1997, 158(1/2):53-92. http://cn.bing.com/academic/profile?id=5481750bfc208874588554042e78fb4a&encoded=0&v=paper_preview&mkt=zh-cn [32] KASZTELAN S, TOULHOAT H, GRIMBLOT J, BONNELLE J P. A geometrical model of the active phase of hydrotreating catalysts[J]. Appl Catal, 1984, 13(1):127-159. http://cn.bing.com/academic/profile?id=60fd24c7f81c487b77b0f94d0cd751e2&encoded=0&v=paper_preview&mkt=zh-cn [33] BRAUN S, APPEL L G, SCHMAL M. Molybdenum species on alumina and silica supports for soot combustion[J]. Catal Commun, 2005, 6(1):7-12. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=6e2a7545056636f0ad02cd673f4a094b [34] SHAHEEN W M. Thermal behaviour of pure and binary basic nickel carbonate and ammonium molybdate systems[J]. Mater Lett, 2002, 52(4/5):272-282. doi: 10.1016-S0167-577X(01)00406-2/ [35] GRIBOVAL A, BLACHARD P, PAYEN E, FOURNIER M, DUBOIS J L. Alumina supported HDS catalysts prepared by impregnation with new heteropolycompounds. Comparison with catalysts prepared by conventional Co-Mo-P coimpregnation[J]. Catal Today, 1998, 45(1/4):277-283. http://cn.bing.com/academic/profile?id=c0360fa0d038c07c86d011ab0a9b6402&encoded=0&v=paper_preview&mkt=zh-cn [36] GRIBOVAL A, BLANCHARD P, GENGEMBRE L, PAYEN E, FOURNIER M, DUBOIS J L, BERNARD J R. Hydrotreatment catalysts prepared with heteropolycompound:Characterization of the oxidic precursors[J]. J Catal, 1999, 188(1):102-110. https://www.deepdyve.com/lp/elsevier/hydrotreatment-catalysts-prepared-with-heteropolycompound-Xvyh4V9ocb [37] SARAVANAN L, SUBRAMANIAN S. Surface chemical studies on the competitive adsorption of poly(ethylene glycol) and ammonium poly(methacrylate) onto alumina[J]. J Colloid Interf Sci, 2005, 284(2):363-377. doi: 10.1016/j.jcis.2004.08.188 [38] PORTELA L, GRANGE P, DELMON B. XPS and NO adsorption studies on alumina-supported Co-Mo catalysts sulfided by different procedures[J]. J Catal, 1995, 156(2):243-254. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=1b794ad7e504130c257994354705a3dc [39] DAMYANOVA S, PETROV L, GRANGE P. XPS characterization of zirconium-promoted CoMo hydrodesulfurization catalysts[J]. Appl Catal A:Gen, 2003, 239(1/2):241-252. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=58d4335aac3b3601c3b40f4f36b83807 [40] ATANASOVA P, HALACHEV T, UCHYTIL J, KRAUS M. Effect of phosphorus on the surface concentration of molybdenum and nickel in the oxide form of nickel-molybdenum-alumina catalysts and on their hydrodesulfurization activity[J]. Appl Catal, 1988, 38:235-240. doi: 10.1016/S0166-9834(00)82828-6 [41] WANG X Q, OZKAN U S. Characterization of active sites over reduced Ni-Mo/Al2O3 catalysts for hydrogenation of linear aldehydes[J]. J Phys Chem B, 2005, 109(5):1882-1890. doi: 10.1021/jp046489q [42] VENEZIA A M, LA PAROLA V, DEGANELLO G, CAUZZI D, LEONARDI G, PREDIERI G. Influence of the preparation method on the thiophene HDS activity of silica supported CoMo catalysts[J]. Appl Catal A:Gen, 2002, 229(1/2):261-271. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=3a7f1dec024570dfa9680b4e96e281ea [43] GUICHARD B, AUBERGER M R, DEVERS E, LEGENS C, RAYBAUD P. Aging of Co(Ni)MoP/Al2O3 catalysts in working state[J]. Catal Today, 2008, 130:97-108. doi: 10.1016/j.cattod.2007.09.007 [44] WALTON R A. Some remarks concerning the X-ray photoelectron spectra of the Co-Mo-Al2O3 hydrodesulfurization catalyst system[J]. J Catal, 1976, 44(2):335-337. http://www.sciencedirect.com/science/article/pii/0021951776904085 [45] PAWELEC B, NAVARRO R M, MARTIN J M C, AGUDO A L, VASUDEVAN P T, FIERRO J L G. Silica-alumina-supported transition metal sulphide catalysts for deep hydrodesulphurization[J]. Catal Today, 2003, 86(1/4):73-85. http://cn.bing.com/academic/profile?id=24a78bc7da30bfa906b9662c00f76d60&encoded=0&v=paper_preview&mkt=zh-cn [46] HAYDEN T F, DUMESIC J A. Studies of the structure of molybdenum oxide and sulfide supported on thin films of alumina[J]. J Catal, 1987, 103(2):366-384. doi: 10.1016/0021-9517(87)90128-X -





 下载:
下载: