Catalytic hydrogenation of furfural to produce 2-methylfuran over Cu/SiO2 catalysts prepared by different silicon sources
-
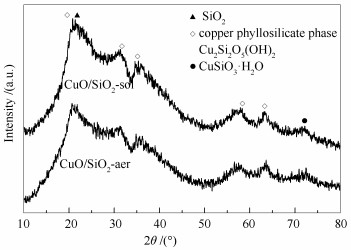
摘要: 以硅溶胶和气相二氧化硅为载体,采用氨蒸法制备了Cu/SiO2-sol和Cu/SiO2-aer两种催化剂,采用N2吸附脱附、X射线衍射(XRD)、傅里叶红外光谱(FT-IR)、透射电子显微镜(TEM)、N2O-H2滴定、氢气程序升温还原(H2-TPR)、氨程序升温脱附(NH3-TPD)及X射线光电子能谱(XPS)对样品进行表征,在固定床反应器中考察两种催化剂对糠醛气相加氢制2-甲基呋喃的催化性能。结果表明,Cu/SiO2-sol催化剂具有更好的催化活性,在150 h反应时间内,糠醛转化率为100%,2-MF选择性在91%以上。这主要归因于以硅溶胶为硅源可以生成更多页硅酸铜,还原后催化剂表面Cu的分散性更高、弱酸位更多,有利于提高糠醛的转化率与2-甲基呋喃的选择性。同时Cu/SiO2-sol具有较大的孔容孔径,有利于降低反应过程中积炭,延长催化剂寿命。Abstract: Both Cu/SiO2-sol and Cu/SiO2-aer catalysts were prepared by the ammonia evaporation method using silicon sol and aerosil as silicon sources, respectively. The catalysts were characterized by N2 adsorption-desorption, XRD, FT-IR, TEM, N2O-H2 titration, H2-TPR, NH3-TPD and XPS. The catalytic performances of Cu/SiO2 catalysts for hydrogenation of furfural to produce 2-methylfuran were investigated in a fixed bed reactor. Compared with Cu/SiO2-aer catalyst, the Cu/SiO2-sol catalyst exhibits a higher catalytic performance. The conversion of furfural is 100% and the selectivity of 2-methylfuran is above 91% during the reaction of 150 h. CuO/SiO2-sol catalyst favors the formation of rich copper phyllosilicate phase, which could provide more weak acid sites and better Cu dispersion on the surface of Cu/SiO2-sol catalyst. Besides, the larger pore size of Cu/SiO2-sol contributes to reducing the carbon deposition, which is beneficial to long service life of the catalyst.
-
Key words:
- Cu/SiO2 /
- support /
- catalysis /
- hydrogenation /
- furfural /
- 2-methylfuran
-
表 1 样品的物理性质测试
Table 1 Physicochemical properties of samples
Sample ABET①/(m2·g-1) vp①/(cm3·g-1) dp①/nm Cu0 dispersion /%② ACu②/(m2·g-1) dCu③/nm Total acidity④/(mmol NH3·gcat-1) SiO2-sol 222 0.299 5.37 - - - - SiO2-aer 344 0.937 10.9 - - - - Cu/SiO2-sol 464 0.969 8.36 26.6 43.8 6.23 0.159 Cu/SiO2-aer 462 0.585 5.06 23.3 37.9 8.07 0.112 Cu/SiO2-sol-used 150 h - - - 23.4 - 13.5 - Cu/SiO2-aer-used 150 h - - - 13.9 - 21.8 - ①: determined using the N2-adsorption method; ②: detected by N2O-H2 titration; ③: average Cu particle size on samples was determined using TEM; ④: the amount of acid sites of reduced samples was determined by NH3-TPD 表 2 Cu/SiO2催化剂催化性能
Table 2 Catalytic performance of the Cu/SiO2 catalysts
Time t/h Cu/SiO2-sol Cu/SiO2-aer x/% 2-MF s/% FOL s/% others s/% x/% 2-MF s/% FOL s/% others s/% 10 100 94.54 0 5.46 100 93.60 0.54 5.86 20 100 93.70 0 6.30 100 92.60 0.64 6.76 50 100 92.78 0 7.22 100 92.50 1.06 6.44 60 100 93.43 0 6.57 100 91.06 2.47 6.47 80 100 92.93 0.10 6.97 100 88.55 5.09 6.36 100 100 92.54 0.67 6.79 99.34 89.82 4.93 5.25 110 100 93.27 0.84 5.89 98.91 85.81 8.16 6.03 120 100 92.26 0.85 6.89 98.56 83.24 11.81 4.95 140 100 91.50 1.23 7.27 96.89 77.91 15.02 7.07 150 100 91.54 1.59 6.87 95.92 76.10 16.07 7.83 2-MF=2-methylfuran, FOL=furfuryl alcohol, others=2-methyltetrahydrofuran, 2-pentanone and 2-pentanol -
[1] MARISCAL R, MAIRELES-TORRES P, OJEDA M, SADABA I, GRANADOS M L. Furfural:A renewable and versatile platform molecule for the synthesis of chemicals and fuels[J]. Energy Environ Sci, 2016, 9(4):1144-1189. doi: 10.1039/C5EE02666K [2] VAN PUTTEN R, VAN DER WAAL J C, DE JONG E, RASRENDRA C B, HEERES H J, DE VRIES J G. Hydroxymethylfurfural, a versatile platform chemical made from renewable resources[J]. Chem Rev, 2013, 113(3):1499-1597. doi: 10.1021/cr300182k [3] PACE V, HOYOS P, CASTOLDI L, DE JONG E, ALCANTARA A R. 2-methyltetrahydrofuran (2-MeTHF):A biomass-derived solvent with broad application in organic chemistry[J]. ChemSusChem, 2012, 5(8):1369-1379. doi: 10.1002/cssc.v5.8 [4] NAKAGAWA Y, TAKADA K, TAMURA M, TOMISHIGE K. Total hydrogenation of furfural and 5-hydroxymethylfurfural over supported Pd-Ir alloy catalyst[J]. Acs Catal, 2014, 4(8):2718-2726. doi: 10.1021/cs500620b [5] YAN K, CHEN A C. Selective hydrogenation of furfural and levulinic acid to biofuels on the ecofriendly Cu-Fe catalyst[J]. Fuel, 2014, 115:101-108. doi: 10.1016/j.fuel.2013.06.042 [6] YAN K, LIAO J Y, WU X, XIE X M. A noble-metal free Cu-catalyst derived from hydrotalcite for highly efficient hydrogenation of biomass-derived furfural and levulinic acid[J]. RSC Adv, 2013, 3(12):3853-3856. doi: 10.1039/c3ra22158j [7] YAN K, CHEN A C. Efficient hydrogenation of biomass-derived furfural and levulinic acid on the facilely synthesized noble-metal-free Cu-Cr catalyst[J]. Energy, 2013, 58:357-363. doi: 10.1016/j.energy.2013.05.035 [8] LANGE J P, VAN DER HEIDE E, PRICE R, VAN BUIJTENEN J, PRICE R. Furfuralu a promising platform for lignocellulosic biofuels[J]. ChemSusChem, 2012, 5(1):150-166. doi: 10.1002/cssc.201100648 [9] ZHENG H Y, ZHU Y L, BAI Z Q, HUANG L, XIANG H W, LI Y W. An environmentally benign process cyclohexanone and 2-methylfuran[J]. Green Chem, 2006, 8(1):107-109. doi: 10.1039/B513584B [10] ZHENG H Y, ZHU Y L, HUANG L, ZENG Z Y, WAN H J, LI Y W. Study on Cu-Mn-Si catalysts for synthesis of cyclohexanone and 2-methylfuran through the coupling process[J]. Catal Commun, 2008, 9(3):342-348. doi: 10.1016/j.catcom.2007.06.026 [11] LESSARD J, MORIN J F, WEHRUNG J F, MAGNIN D, CHORNET E. High yield conversion of residual pentoses into furfural via zeolite catalysis and catalytic hydrogenation of furfural to 2-methylfuran[J]. Top Catal, 2010, 53(15/18):1231-1234. http://cn.bing.com/academic/profile?id=b040b09bb605c1a915bbf58a1e1c25d4&encoded=0&v=paper_preview&mkt=zh-cn [12] CHANG X, LIU A F, CAI B, LUO J C, WU W B, YU J T. Catalytic transfer hydrogenation of furfural to 2-methylfuran and 2-methyltetrahydrofuran over bimetallic copper-palladium catalysts[J]. ChemSusChem, 2016, 9(23):3330-3337. doi: 10.1002/cssc.v9.23 [13] HUTCHINGS G S, LUC W, LU Q, ZHOU Y, VLACHOS D G, JIAO F. Nanoporous Cu-Al-Co alloys for selective furfural hydrodeoxygenation to 2-methylfuran[J]. Ind Eng Chem Res, 2017, 56(14):3866-3872. doi: 10.1021/acs.iecr.7b00316 [14] POPA T, ZHANG Y L, JIN E L, FAN M H. An environmentally benign and low-cost approach to synthesis of thermally stable industrial catalyst Cu/SiO2 for the hydrogenation of dimethyl oxalate to ethylene glycol[J]. Appl Catal A:Gen, 2015, 505:52-61. doi: 10.1016/j.apcata.2015.07.026 [15] DING T M, TIAN H S, LIU J C, WU W B, YU J T. Highly active Cu/SiO2 catalysts for hydrogenation of diethyl malonate to 1, 3-propanediol[J]. Chin J Catal, 2016. 37(4):484-493. doi: 10.1016/S1872-2067(15)61053-1 [16] LI F, LU C S, LI X N. The effect of the amount of ammonia on the Cu0/Cu+ ratio of Cu/SiO2 catalyst for the hydrogenation of dimethyl oxalate to ethylene glycol[J]. Chin Chem Lett, 2014, 25(11):1461-1465. doi: 10.1016/j.cclet.2014.05.050 [17] YIN A Y, GUO X Y, DAI W L, FAN K N. Effect of initial precipitation temperature on the structural evolution and catalytic behavior of Cu/SiO2 catalyst in the hydrogenation of dimethyloxalate[J]. Catal Commun, 2011, 12(6):412-416. doi: 10.1016/j.catcom.2010.10.030 [18] PERNICONE N, FANTINEL T, BALDAN C, RIELLO P, PINNA F. On the measurement of copper surface area by oxygen chemisorption[J]. Appl Catal A:Gen, 2003, 240(1/2):199-206. http://cn.bing.com/academic/profile?id=1ef33ed1fbc709e149fe3e4347142900&encoded=0&v=paper_preview&mkt=zh-cn [19] HUANG Z W, CUI F, XUE J J, ZUO J L, CHEN J, XIA C G. Cu/SiO2 catalysts prepared by hom-and heterogeneous deposition-precipitation methods:Texture, structure, and catalytic performance in the hydrogenolysis of glycerol to 1, 2-propanediol[J]. Catal Today, 2012, 183(1):42-51. doi: 10.1016/j.cattod.2011.08.038 [20] ZHANG C C, WANG D H, ZHU M Y, YU F, DAI B. Effect of different nano-sized silica sols as supports on the structure and properties of Cu/SiO2 for hydrogenation of dimethyl oxalate[J]. Catalysts, 2017, 7(3):12. https://pdfs.semanticscholar.org/1621/5a2636053a14a088b8d824850a75f8f50d1b.pdf [21] GONG J L, YUE H R, ZHAO Y J, ZHAO S, ZHAO L, LV J, WANG S P, MA X B. Synthesis of ethanol via syngas on Cu/SiO2 catalysts with balanced Cu0/Cu+sites[J]. J Am Chem Soc, 2012, 134(34):13922-13925. doi: 10.1021/ja3034153 [22] DI W, CHENG J H, TIAN S X, LI J, CHEN L F, ZHU Y L, LIA Y W. Synthesis and characterization of supported copper phyllosilicate catalysts for acetic ester hydrogenation to ethanol[J]. Appl Catal A:Gen, 2016. 510:244-259. doi: 10.1016/j.apcata.2015.10.026 [23] 王新雷, 马奎, 郭丽红, 丁彤, 程庆鹏, 田野, 李新刚.蒸氨法制备铜硅催化剂的二甲醚水蒸气重整制氢性能[J].物理化学学报, 2017, 33(8):1699-1708. doi: 10.3866/PKU.WHXB201704263WANG Xin-lei, MA Kui, GUO Li-hong, DING Tong, CHENG Qing-peng, TIAN Ye, LI Xin-gang. Catalytic performance for hydrogen production through steam reforming of dimethyl ether over silica supported copper catalysts synthesized by ammonia evaporation method[J]. Acta Phys Chim Sin, 2017, 33(8):1699-1708. doi: 10.3866/PKU.WHXB201704263 [24] 贺黎明, 陈晓春, 何海龙, 马京生, 王伟, 郝玉春.溶胶-凝胶法制备Cu/SiO2催化剂的表征与性能[J].石油化工, 2010, 39(12):1337-1343. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=syhg201012026&dbname=CJFD&dbcode=CJFQHE Li-ming, CHEN Xiao-chun, HE Hai-long, MA Jing-sheng, WANG Wei, HAO Yu-chun. Characteirzations and catalytic performances of Cu/SiO2 catalysts prepared by sol gel mehtod[J]. Petro Chem Technol, 2010, 39(12):1337-1343. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=syhg201012026&dbname=CJFD&dbcode=CJFQ [25] DONG F, ZHU Y L, ZHENG H Y, ZHU Y F, LI X Q, LI Y W. Cr-free Cu-catalysts for the selective hydrogenation of biomass-derived furfural to 2-methylfuran:The synergistic effect of metal and acid sites[J]. J Mol Catal A:Chem, 2015, 398:140-148. doi: 10.1016/j.molcata.2014.12.001 [26] YIN A Y, GUO X Y, DAI W L, FAN K N. The nature of active copper species in cu-hms catalyst for hydrogenation of dimethyl oxalate to ethylene glycol:New insights on the synergetic effect between Cu0 and Cu+[J]. J Phys Chem C, 2009, 113(25):11003-11013. doi: 10.1021/jp902688b [27] YANG X H, XIANG X M, CHEN H M, ZHENG H Y, LI Y W, ZHU Y L. Efficient synthesis of furfuryl alcohol and 2-methylfuran from furfural over mineral-derived Cu/ZnO catalysts[J]. ChemCatChem, 2017, 9(15):3023-3030. doi: 10.1002/cctc.v9.15 [28] 黄玉辉, 任国卿, 孙蛟, 陈晓蓉, 梅华. Cu/ZnO催化糠醛气相加氢制2-甲基呋喃的研究[J].燃料化学学报, 2016, 44(11):1349-1355. doi: 10.3969/j.issn.0253-2409.2016.11.011HUANG Yu-hui, REN Guo-qing, SUN Jiao, CHEN Xiao-rong, MEI Hua. Study on the vapor phase hydrogenation of furfural to 2-methylfuran on Cu/ZnO catalyst[J]. J Fuel Chem Technol, 2016, 44(11):1349-1355. doi: 10.3969/j.issn.0253-2409.2016.11.011 -





 下载:
下载: