Influence of Cu and Mo components of γ-Al2O3 supported nickel catalysts on hydrodeoxygenation of fatty acid methyl esters to fuel-like hydrocarbons
-
摘要: 制备一系列包含或不包含铜、钼组分的Ni/γ-Al2O3催化剂,并对其进行表征和性能测试。考察了铜、钼负载量,浸渍顺序(包括连续浸渍和共浸渍),反应条件对脂肪酸甲酯加氢脱氧反应性能的影响。根据TG数据,使用过的20Ni-6Cu/γ-Al2O3催化剂其热失重小于20Ni/γ-Al2O3催化剂,这表明,铜的引入能够有效抑制反应过程中催化剂表面的积炭行为。对于20Ni-6Cu/γ-Al2O3和20Ni-6Cu-nMo/γ-Al2O3(n=2、5、8和12)催化剂,NH3-TPD分析结果显示,钼物相的引入对载体γ-Al2O3的酸性位有着显著影响,当钼负载量达到5%时,可以观察到一个新的酸位对应于中强酸位。铜和钼修饰过的催化剂其催化性能要高于Ni/γ-Al2O3催化剂。从XPS的分析可以看出,催化剂中的铜主要以正二价形式存在,钼主要以正四价和正六价形式存在,而且不同的浸渍顺序会影响催化剂表面活性组分的实际含量。此外,脂肪酸甲酯的转化率和烷烃产品的收率也和所制备出来的催化剂的浸渍顺序有关。在所有的催化剂中,使用连续浸渍(先浸渍镍铜组分、浸渍钼组分)所制备的三金属20Ni-6Cu-5Mo/γ-Al2O3催化剂展现了优异的催化性能。在适宜的反应条件下(350 ℃,2.5 MPa,WSHV=2.0 h-1,H2/oil ratio=1250 mL/mL),脂肪酸甲酯的转化率和烷烃产品的收率分别达到98.4%和94.2%。Abstract: In this work, series of Ni/γ-Al2O3 catalysts with or without Cu and Mo components were prepared, characterized and tested for the hydrodeoxygenation of fatty acid methyl esters (FAME) to hydrocarbons. The effects of Cu and Mo loading, impregnation sequence with sequential impregnation and co-impregnation involved and reaction conditions on catalytic performance were investigated. According to the TG data, the spent 20Ni-6Cu/γ-Al2O3 catalyst gives less weight loss than the spent 20Ni/γ-Al2O3 catalyst, which indicates that the addition of copper inhibits the carbon deposits behavior on catalyst surface during reaction. For 20Ni-6Cu/γ-Al2O3 and 20Ni-6Cu-nMo/γ-Al2O3(n=2, 5, 8 and 12) catalysts, NH3-TPD analysis shows that the addition of molybdenum phase has significant impact on the acid sites of γ-Al2O3. When the loading of molybdenum is 5%, a new acid site is observed corresponding to the medium strength acid site. The Cu and Mo modified catalysts demonstrate better catalytic performance than Ni/γ-Al2O3 catalysts. Based on XPS results, Cu in the catalysts exists in the Cu2+ and Mo in the catalysts has two states:Mo4+ and Mo6+, and different impregnation sequences may influence the actual element composition of active phase on the surface of catalysts. In addition, the conversion of FAME and yield of alkane are related to the catalysts prepared with different impregnation sequence. Among all the catalysts, the trimetallic 20Ni-6Cu-5Mo/γ-Al2O3 prepared by sequential impregnation (the former Ni and Cu impregnation, the latter Mo impregnation) exhibits optimal catalytic performance. Under appropriate reaction conditions 350℃, 2.5 MPa, WSHV=2.0 h-1, H2/oil ratio=1250 mL/mL, the conversion of FAME and yield of alkane are 98.4% and 94.2%.
-
Key words:
- hydrodeoxygenation /
- fatty acid methyl esters /
- Ni-based catalysts /
- γ-Al2O3
-
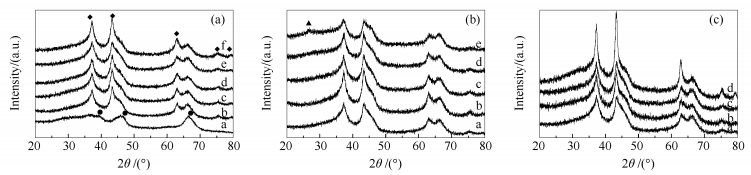
Figure 1 XRD patterns of γ-Al2O3 and catalysts
(a): a: γ-Al2O3; b: 20Ni/γ-Al2O3; c: 20Ni-1Cu/γ-Al2O3; d: 20Ni-3Cu/γ-Al2O3; e: 20Ni-6Cu/γ-Al2O3; f: 20Ni-10Cu/γ-Al2O3
(b): a: 20Ni-6Cu/γ-Al2O3; b: 20Ni-6Cu-2Mo/γ-Al2O3; c: 20Ni-6Cu-5Mo/γ-Al2O3; d: 20Ni-6Cu-8Mo/γ-Al2O3; e: 20Ni-6Cu-12Mo/γ-Al2O3
(c): a: 20Ni-6Cu/γ-Al2O3; b: Ni-Cu-Mo(s)/γ-Al2O3; c: Ni-Cu-Mo/γ-Al2O3; d: Mo-Ni-Cu/γ-Al2O3
(a): ◆: NiO; ●: Al2O3; (b): ▲: MoO3Figure 4 H2-TPR profiles of catalysts
(a): a: 20Ni/γ-Al2O3; b: 20Ni-1Cu/γ-Al2O3; c: 20Ni-3Cu/γ-Al2O3; d: 20Ni-6Cu/γ-Al2O3; e: 20Ni-10Cu/γ-Al2O3; f: 6Ni-20Cu/γ-Al2O3
(b): a: 20Ni-6Cu/γ-Al2O3; b: 20Ni-6Cu-2Mo/γ-Al2O3; c: 20Ni-6Cu-5Mo/γ-Al2O3; d: 20Ni-6Cu-8Mo/γ-Al2O3; e: 20Ni-6Cu-12Mo/γ-Al2O3
(c): a: 20Ni-6Cu/γ-Al2O3; b: Ni-Cu-Mo(s)/γ-Al2O3; c: Mo-Ni-Cu/γ-Al2O3; d: Ni-Cu-Mo/γ-Al2O3Figure 5 NH3-TPD profiles of γ-Al2O3 and catalysts
(a): a: γ-Al2O3; b: 20Ni/γ-Al2O3; c: 20Ni-1Cu/γ-Al2O3; d: 20Ni-3Cu/γ-Al2O3; e: 20Ni-6Cu/γ-Al2O3; f: 20Ni-10Cu/γ-Al2O3
(b): a: 20Ni-6Cu/γ-Al2O3; b: 20Ni-6Cu-2Mo/γ-Al2O3; c: 20Ni-6Cu-5Mo/γ-Al2O3; d: 20Ni-6Cu-8Mo/γ-Al2O3; e: 20Ni-6Cu-12Mo/γ-Al2O3
(c): a: 20Ni-6Cu/γ-Al2O3; b: Ni-Cu-Mo(s)/γ-Al2O3; c: Mo-Ni-Cu/γ-Al2O3; d: Ni-Cu-Mo/γ-Al2O3Table 1 Textural properties of the catalysts
Catalyst BET surface area A/(m2·g-1) Pore volume v/(cm3·g-1) Avg. pore diameter d/nm 20Ni/γ-Al2O3 278 0.68 4.88 20Ni-1Cu/γ-Al2O3 271 0.64 4.89 20Ni-3Cu/γ-Al2O3 242 0.62 4.88 20Ni-6Cu/γ-Al2O3 222 0.47 4.90 20Ni-10Cu/γ-Al2O3 203 0.44 4.90 20Ni-6Cu-2Mo/γ-Al2O3 212 0.45 4.89 20Ni-6Cu-5Mo/γ-Al2O3 207 0.43 4.89 20Ni-6Cu-8Mo/γ-Al2O3 204 0.41 4.31 20Ni-6Cu-12Mo/γ-Al2O3 188 0.31 3.82 Ni-Cu-Mo(s)/γ-Al2O3 214 0.45 4.89 Mo-Ni-Cu/γ-Al2O3 213 0.43 4.31 Table 2 Relative atomic ratios of elements in the near-surface layer of catalysts with different Mo contents
Catalyst [Ni]/[Al] [Cu]/[Al] [Mo]/[Al] [O]/[Al] 20Ni-6Cu/γ-Al2O3 0.05 0.006 - 1.04 20Ni-6Cu-2Mo/γ-Al2O3 0.05 0.006 0.01 1.17 20Ni-6Cu-5Mo/γ-Al2O3 0.05 0.006 0.03 1.14 20Ni-6Cu-8Mo/γ-Al2O3 0.04 0.007 0.04 1.20 20Ni-6Cu-12Mo/γ-Al2O3 0.06 0.008 0.07 1.47 Table 3 Relative atomic ratios of elements in the near-surface layer of catalysts from different impregnation sequence
Catalyst [Ni]/[Al] [Cu]/[Al] [Mo]/[Al] [O]/[Al] Ni-Cu-Mo/γ-Al2O3 0.05 0.006 0.03 1.14 Mo-Ni-Cu/γ-Al2O3 0.04 0.006 0.02 1.16 Ni-Cu-Mo(s)/γ-Al2O3 0.04 0.005 0.02 1.12 Table 4 Contents of various elements of γ-Al2O3, 20Ni-6Cu/γ-Al2O3 and 20Ni-6Cu-5Mo/γ-Al2O3catalysts
Sample Elemental composition w/% O Al Ni Cu Mo γ-Al2O3 56.17 43.83 20Ni-6Cu/γ-Al2O3 48.89 27.53 17.99 5.59 20Ni-6Cu-5Mo/γ-Al2O3 46.30 26.72 17.44 4.67 4.87 -
[1] KUMAR R, FAROOQUI S A, ANAND M, KUMAR R, JOSHI R, KHAN A, SINHA A K. Hydrotreatment of jatropha oil over NiMoS catalyst supported on thermostable mesoporous silica doped titania for the production of renewable drop-in diesel[J]. Catal Commun, 2017, 98:102-106. doi: 10.1016/j.catcom.2017.04.047 [2] ZHANG Z N, TANG M X, CHEN J X. Effects of P/Ni ratio and Ni content on performance of γ-Al2O3-supported nickel phosphides for deoxygenation of methyl laurate to hydrocarbons[J]. Appl Surf Sci, 2016, 360(4):353-364. doi: 10.1016/j.apsusc.2015.10.182 [3] LOE R, SANTILLAN-JIMENEZ E, MORGAN T, SEWELL L, JI Y, JONES S, ISAACS M A, LEE A F, CROCKER M. Effect of Cu and Sn promotion on the catalytic deoxygenation of model and algal lipids to fuel-like hydrocarbons over supported Ni catalysts[J]. Appl Catal B:Environ, 2016, 191:147-156. doi: 10.1016/j.apcatb.2016.03.025 [4] ROH H S, EUM I H, JEONG D W, YI B E, NA J G, KO C H. The effect of calcination temperature on the performance of Ni/MgO-Al2O3 catalysts for decarboxylation of oleic acid[J]. Catal Today, 2011, 164(1):457-460. doi: 10.1016/j.cattod.2010.10.048 [5] LIU Q Y, ZUO H L, WANG T J, MA L L, ZHANG Q. One-step hydrodeoxygenation of palm oil to isomerized hydrocarbon fuels over Ni supported on nano-sized SAPO-11 catalysts[J]. Appl Catal A:Gen, 2013, 468(12):68-74. https://www.researchgate.net/publication/269924108_One-step_hydrodeoxygenation_of_palm_oil_to_isomerized_hydrocarbon_fuels_over_Ni_supported_on_nano-sized_SAPO-11_catalysts [6] XIN H, GUO K, LI D, YANG H Q, HU C W. Production of high-grade diesel from palmitic acid over activated carbon-supported nickel phosphide catalysts[J]. Appl Catal B:Environ, 2016, 187:375-385. doi: 10.1016/j.apcatb.2016.01.051 [7] KUKUSHKIN R G, BULAVCHENKO O A, KAICHEV V V, YAKOVLEV V A. Influence of Mo on catalytic activity of Ni-based catalysts in hydrodeoxygenation of esters[J]. Appl Catal B:Environ, 2015, 163:531-538. doi: 10.1016/j.apcatb.2014.08.001 [8] ZHAO S, ZHANG Z N, ZHU K Y, CHEN J X. Hydroconversion of methyl laurate on bifunctional Ni2P/AlMCM-41 catalyst prepared via in situ phosphorization using triphenylphosphine[J]. Appl Surf Sci, 2017, 404:388-397. doi: 10.1016/j.apsusc.2017.02.016 [9] CHEN N, GONG S F, QIAN E W. Effect of reduction temperature of NiMoO3-x/SAPO-11 on its catalytic activity in hydrodeoxygenation of methyl laurate[J]. Appl Catal B:Environ, 2015, 174/175:253-263. doi: 10.1016/j.apcatb.2015.03.011 [10] SHI H, CHEN J X, YANG Y, TIAN S S. Catalytic deoxygenation of methyl laurate as a model compound to hydrocarbons on nickel phosphide catalysts:Remarkable support effect[J]. Fuel Process Technol, 2014, 118(1):161-170. https://www.sciencedirect.com/science/article/pii/S0378382013002713 [11] CHEN J X, YANG Y, SHI H, LI M F, CHU Y, PAN Z Y, YU X B. Regulating product distribution in deoxygenation of methyl laurate on silica-supported Ni-Mo phosphides:Effect of Ni/Mo ratio[J]. Fuel, 2014, 129(7):1-10. https://www.researchgate.net/publication/272027839_Deoxygenation_of_methyl_laurate_to_hydrocarbons_on_silica-supported_Ni-Mo_phosphides_Effect_of_calcination_temperatures_of_precursor [12] JENIŠTOVÁ K, HACHEMI I, MÄKI-ARVELA P, KUMAR N, PEURLA M, ČAPEK L, WÄRNÅ J, MURZIN D Y. Hydrodeoxygenation of stearic acid and tall oil fatty acids over Ni-alumina catalysts:Influence of reaction parameters and kinetic modelling[J]. Chem Eng J, 2017, 316:401-409. doi: 10.1016/j.cej.2017.01.117 [13] ZUO H L, LIU Q Y, WANG T J, MA L L, ZHANG Q, ZHANG Q. Hydrodeoxygenation of methyl palmitate over supported Ni catalysts for diesel-like fuel production[J]. Energy Fuels, 2012, 26(6):3747-3755. doi: 10.1021/ef300063b [14] KORDULIS C, BOURIKAS K, GOUSI M, KORDOULI E, LYCOURGHIOTIS A. Development of nickel based catalysts for the transformation of natural triglycerides and related compounds into green diesel:A critical review[J]. Appl Catal B:Environ, 2016, 181:156-196. doi: 10.1016/j.apcatb.2015.07.042 [15] GALEA N M, KNAPP D, ZIEGLER T. Density functional theory studies of methane dissociation on anode catalysts in solid-oxide fuel cells:Suggestions for coke reduction[J]. J Catal, 2007, 247(1):20-33. doi: 10.1016/j.jcat.2006.12.021 [16] FIERRO V, AKDIM O, MIRODATOS C. On-board hydrogen production in a hybrid electric vehicle by bio-ethanol oxidative steam reforming over Ni and noble metal based catalysts[J]. Green Chem, 2003, 5(1):20-24. doi: 10.1039/b208201m [17] BOUDJAHEM A G, CHETTIBI M, MONTEVERDI S, BETTAHAR M M. Acetylene hydrogenation over Ni-Cu nanoparticles supported on silica prepared by aqueous hydrazine reduction[J]. J Nanosci Nanotechnol, 2009, 9(6):3546-3554. doi: 10.1166/jnn.2009.NS28 [18] GUO Q, WU M, WANG K, ZHANG L, XU X. Catalytic hydrodeoxygenation of algae bio-oil over bimetallic Ni-Cu/ZrO2, catalysts[J]. Ind Eng Chem Res, 2015, 54(3):890-899. doi: 10.1021/ie5042935 [19] ARDIYANTI A R, KHROMOVA S A, VENDERBOSCH R H, YAKOVLEV V A, MELIÁN-CABRERA I V, HEERES H J. Catalytic hydrotreatment of fast pyrolysis oil using bimetallic Ni-Cu catalysts on various supports[J]. Appl Catal A:Gen, 2012, 449:121-130. doi: 10.1016/j.apcata.2012.09.016 [20] MICHIO A, HARUO T, KIYOSHI O, KUNIO S, TADASUKE H, NAOYUKI T. Thermally stable nickel-molybdenum alloy catalysts supported on magnesium aluminate for high temperature methanation[J]. Sekiyu Gakkaishi, 2008, 24(6):363-370. https://www.researchgate.net/publication/271874895_Methanation_Activity_of_Tungsten_Catalysts_in_the_Presence_of_Hydrogen_Sulfide_at_an_Elevated_Temperature [21] KADINOV G, PRALIAUD H, PRIMET M, MARTIN G A. Morphological, electronic and catalytic properties of silica-supported nickel and nickel-molybdenum catalysts[J]. Appl Catal, 1984, 10(1):63-76. doi: 10.1016/0166-9834(84)85006-X [22] AGUADO J, ESCOLA J M, CASTRO M C. Influence of the thermal treatment upon the textural properties of sol-gel mesoporousγ-alumina synthesized with cationic surfactants[J]. Microporous Mesoporous Mater, 2010, 128(1/3):48-55. doi: 10.1007/s40097-016-0192-3 [23] LIU J, LIU C, ZHOU G, SHEN S T, RONG L. Hydrotreatment of Jatropha oil over NiMoLa/Al2O3 catalyst[J]. Green Chem, 2012, 14(9):2499-2505. doi: 10.1039/c2gc35450k [24] THOMMES M, KANEKO K, NEIMARK A V, OLIVIER J P, RODRIGUEZ-REINOSO F, ROUQUEROL J, SING K S W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report)[J]. Pure Appl Chem, 2011, 38(1):25-25. http://sol.rutgers.edu/~aneimark/PDFs/IUPAC_Report_PAC_2015.pdf [25] LIU C Y, YANG H, JING Z Y, XI K Z, QIAO C Z. Hydrodeoxygenation of fatty acid methyl esters and isomerization of products over NiP/SAPO-11 catalysts[J]. Fuel Chem Technol, 2016, 44(10):1211-1216. doi: 10.1016/S1872-5813(16)30052-4 [26] LIU Q Y, ZUO H L, ZHANG Q, WANG T J, MA L L. Hydrodeoxygenation of palm oil to hydrocarbon fuels over Ni/SAPO-11 catalysts[J]. Chin J Catal, 2014, 35(5):748-756. doi: 10.1016/S1872-2067(12)60710-4 [27] TIAN S S, CHEN J X, . Hydroisomerization of n-dodecane on a new kind of bifunctional catalyst:Nickel phosphide supported on SAPO-11 molecular sieve[J]. Fuel Process Technol, 2014, 122(122):120-128. https://www.researchgate.net/publication/260212317_Hydroisomerization_of_n-dodecane_on_a_new_kind_of_bifunctional_catalyst_Nickel_phosphide_supported_on_SAPO-11_molecular_sieve [28] ASSAF P G M, NOGUEIRA F G E, ASSAF E M. Ni and Co catalysts supported on alumina applied to steam reforming of acetic acid:Representative compound for the aqueous phase of bio-oil derived from biomass[J]. Catal Today, 2013, 213(37):2-8. https://www.researchgate.net/publication/257325303_Ni_and_Co_catalysts_supported_on_alumina_applied_to_steam_reforming_of_acetic_acid_Representative_compound_for_the_aqueous_phase_of_bio-oil_derived_from_biomass [29] BERTEAU P, DELMON B. Modified aluminas:Relationship between activity in 1-butanol dehydration and acidity measured by NH3-TPD[J]. Catal Today, 1989, 5(2):121-137. doi: 10.1016/0920-5861(89)80020-3 [30] ZHAO S, LI M F, CHU Y, CHEN J X. Hydroconversion of methyl laurate as a model compound to hydrocarbons on bifunctional Ni2P/SAPO-11:Simultaneous comparison with the performance of Ni/SAPO-11[J]. Energy Fuels, 2014, 28(11):7122-7132. doi: 10.1021/ef501723p [31] XIA Z J, LIU H Y, LU H F, ZHANG Z K, CHEN Y F. Study on catalytic properties and carbon deposition of Ni-Cu/SBA-15 for cyclohexane dehydrogenation[J]. Appl Surf Sci, 2017, 422:905-912. doi: 10.1016/j.apsusc.2017.04.245 [32] KHROMOVA S A, SMIRNOV A A, BULAVCHENKO O A, SARAEV A A, KAICHEV V V, Reshetnikov S I, YAKOVLEV V A. Anisole hydrodeoxygenation over Ni-Cu bimetallic catalysts:The effect of Ni/Cu ratio on selectivity[J]. Appl Catal A:Gen, 2014, 470(2):261-270. https://www.sciencedirect.com/science/article/pii/S0926860X13006637 [33] KOCHETKOVA D, BLAŽEK J, ŠIMÁČEK P, STAŠ M, BEŇO Z. Influence of rapeseed oil hydrotreating on hydrogenation activity of CoMo catalyst[J]. Fuel Process Technol, 2016, 142:319-325. doi: 10.1016/j.fuproc.2015.10.034 [34] BOTAS J A, SERRANO D P, GARCÍA A, VICENTE J D, RAMOSA R. Catalytic conversion of rapeseed oil into raw chemicals and fuels over Ni-and Mo-modified nanocrystalline ZSM-5 zeolite[J]. Catal Today, 2012, 195(1):59-70. doi: 10.1016/j.cattod.2012.04.061 [35] TOBAA M, ABEA Y, KURAMOCHI H, OSAKO M, MOCHIZUKI T, YOSHIMURAA Y J. Hydrodeoxygenation of waste vegetable oil over sulfide catalysts[J]. Catal Today, 2011, 164(1):533-537. doi: 10.1016/j.cattod.2010.11.049 [36] VERMA D, RANA B S, KUMAR R, SIBI, M G, SINHA A K. Diesel and aviation kerosene with desired aromatics from hydroprocessing of jatropha oil over hydrogenation catalysts supported on hierarchical mesoporous SAPO-11[J]. Appl Catal A:Gen, 2015, 490:108-116. doi: 10.1016/j.apcata.2014.11.007 [37] PERONI M, MANCINO G, BARÁTHA E, GUTIÉRREZ O Y, LERCHER J A. Bulk andγAl2O3-supported Ni2P and MoP for hydrodeoxygenation of palmitic acid[J]. Appl Catal B:Environ, 2016, 180:301-311. doi: 10.1016/j.apcatb.2015.06.042 [38] WANG H Y, JIAO T T, LI Z X, LI C S, ZHANG S J, ZHANG J L. Study on palm oil hydrogenation for clean fuel over Ni-Mo-W/γ-Al2O3-ZSM-5 catalyst[J]. Fuel Process Technol, 2015, 139:91-99. doi: 10.1016/j.fuproc.2015.08.004 -





 下载:
下载: