Study on the vapor phase hydrogenation of furfural to 2-methylfuran on Cu/ZnO catalyst
-
摘要: 通过共沉淀法制备一系列铜锌催化剂,用于固定床上糠醛气相加氢制2-甲基呋喃的研究。采用X射线衍射仪(XRD)、N2吸附-脱附、扫描电子显微镜(SEM)、H2-程序升温还原(H2-TPR)、NH3-程序升温脱附(NH3-TPD)表征,分析催化剂中Cu0和ZnO在催化反应中的作用。结果表明,Cu0是糠醛加氢的活性中心,氧化锌的加入减小了催化剂晶粒粒径、增大了催化剂比表面积、利于催化剂还原和增加催化剂表面弱酸性位。当Cu/Zn物质的量比为1:2时,Cu1Zn2催化剂具有适宜氧化还原活性中心及弱酸位数量,对2-甲基呋喃表现出较高的选择性。Cu1Zn2催化剂在常压、反应温度为200℃、氢醛物质的量比为4:1、糠醛体积空速为0.3 h-1条件下,糠醛转化率100.0%,2-甲基呋喃选择性最高为93.6%。反应稳定运行200 h后,糠醛转化率仍为100.0%,2-甲基呋喃选择性为80.0%,糠醇选择性为11.4%。Abstract: A series of Cu/ZnO catalysts were prepared by using co-precipitation method and their performance for the furfural gas phase hydrogenation to 2-methylfuran was investigated in a fixed bed reactor. The catalysts were characterized by X-ray diffraction (XRD), N2 adsorption desorption, H2 temperature programmed reduction (H2-TPR), scanning electron microscope (SEM) and NH3-temperature programmed desorption (NH3-TPD) techniques to analyze the roles of Cu0 and ZnO. The results showed that Cu0 was the active center for the furfural hydrogenation and the addition of ZnO in Cu catalysts can reduce the crystal size, enhance the surface area, improve the reduction and increase the surface acidity of the catalysts. When the molar ratio of Cu/Zn molar ratio is 1:2, Cu1Zn2 catalyst showed the highest selectivity to 2-methylfuran due to its suitable numbers of redox active centers and weak acidic sites. Under the atmospheric pressure, reaction temperature of 200℃, 4:1 molar ratio of hydrogen to furfural and furfural volume space velocity of 0.3 h-1, the conversion of furfural reached almost 100.0% with 93.6% selectivity to 2-methylfuran over Cu1Zn2 catalyst.
-
Key words:
- co-precipitation method /
- Cu/ZnO catalyst /
- furfural /
- gas hydrogenation /
- 2-methylfuran
-
表 1 铜锌催化剂的比表面积及还原前后的晶粒粒径
Table 1 BET analysis, crystal sizes of calcined and reduced Cu/ZnO catalysts
Catalyst ABET /(m2·g-1) Crystal size d/nm calcined sample reduced sample CuO ZnO Cu0 ZnO Cu1Zn4 40.6 10.4 14.6 13.4 16.4 Cu1Zn2 41.7 11.1 13.5 16.4 14.4 Cu1Zn1 38.0 11.2 12.0 16.7 18.2 Cu1Zn0.67 25.0 12.1 11.9 17.4 22.4 Cu1Zn0 6.0 14.8 - 28.6 - 表 2 铜锌催化剂的性能评价
Table 2 Evaluation of catalytic performance over Cu/ZnO
Catalyst FFR
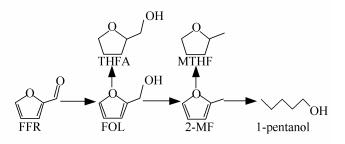
conversion x/%Product selectivity s/% 2-MF FOL THFA MTHF 1-pentanol others Cu1Zn4 100.0 88.7 4.1 - 0.8 0.4 6.0 Cu1Zn2 100.0 93.6 0.5 - 1.4 0.6 3.9 Cu1Zn1 100.0 92.0 - - 2.3 1.8 3.9 Cu1Zn0.67 100.0 87.9 - - 3.3 3.3 5.5 Cu1Zn0 99.2 8.8 86.4 2.5 0.4 - 1.9 -
[1] VAN P, R J, JCV D W, DE J E. Hydroxymethylfurfural, a versatile platform chemical made from renewable resources[J]. Chem Rev, 2013, 113(3):1499-1597. doi: 10.1021/cr300182k [2] BINDER J B, RAINES R T. Simple chemical transformation of lignocellulosic biomass into furans for fuels and chemicals[J]. J Am Chem Soc, 2009, 131(5):1979-1985. doi: 10.1021/ja808537j [3] MAMMAN A S, LEE J M, KIM Y C. Furfural:Hemicellulose/xylosederived biochemical[J]. Biofuel Bioprod Bior, 2008, 2(5):438-454. doi: 10.1002/bbb.v2:5 [4] PACE V, HOYOS P, CASTOLDI L. ChemInform abstract:2-methyltetrahydrofuran (2-MeTHF):A biomass-derived solvent with broad application in organic chemistry[J]. ChemSusChem, 2012, 5(8):1369-1379. doi: 10.1002/cssc.v5.8 [5] BIRADAR N S, HENNGNE A M, BIRAJDAR S N. Single-pot formation of THFAL via catalytic hydrogenation of FFR over Pd/MFI catalyst[J]. Acs Sustainable Chem Eng, 2013, 2(2):272-281. https://www.researchgate.net/profile/Narayan_Biradar/publication/258993551_Single-pot_formation_of_THFAL_via_catalytic_hydrogenation_of_FFR_over_PdMFI_catalyst/links/02e7e5298c86842219000000.pdf?inViewer=true&pdfJsDownload=true&disableCoverPage=true&origin=publication_detail [6] HUBER G W, SARA I A, CORMA A. Synthesis of transportation fuels from biomass:Chemistry, catalysts, and engineering[J]. Chem Rev, 2006, 106(9):4044-4498. doi: 10.1021/cr068360d [7] XIAO M, JING C, XU H. Laminar burning characteristics of 2-methylfuran and isooctane blend fuels[J]. Fuel, 2014, 116(1):281-291. https://www.researchgate.net/profile/Hongming_Xu3/publication/262937481_Laminar_burning_characteristics_of_2-methylfuran_and_isooctane_blend/links/56efd74808ae3c65343653e3.pdf?origin=publication_detail [8] BURNETTL W, JOHNS I B, HOLDREN R F. Production of 2-methylfuran by vapor-phase hydrogenation of furfural[J]. Ind Eng Chem, 2002, 74(2):129-130. https://www.researchgate.net/publication/231384595_Production_of_2-Methylfuran_by_Vapor-Phase_Hydrogenation_of_Furfural [9] 吴静, 申延明, 王坤院. CuO-CaO/SiO2超细催化剂结构及糠醛加氢反应性能的研究[J].分子催化, 2003, 17(5):321-325. http://www.oalib.com/paper/4480393WU Jing, SHEN Yan-ming, WANG Kun-yuan. Study on structure of CuO-CaO/SiO2 ultrafine catalysts and reaction performance for hydrogenation of furfural[J]. J Mol Catal, 2003, 17(5):321-325. http://www.oalib.com/paper/4480393 [10] 苗小培, 冯海强, 黄文氢.纳米级CuO催化剂的制备及其糠醛加氢催化性能[J].石油化工, 2015, 44(8):975-979. http://d.wanfangdata.com.cn/Periodical/syhg201508012MIAO Xiao-pei, FENG Hai-qiang, HUANG Wen-qing. Preparation and catalytic properties of nanometer CuO catalyst for hydrogena[J]. Petrochem Technol, 2015, 44(8):975-999. http://d.wanfangdata.com.cn/Periodical/syhg201508012 [11] KAI Y, XU W, XIA A, XIAN M X. Novel preparation of nano-composite CuO-Cr2O3 using ctab-template method and efficient for hydrogenation of biomass-derived furfural[J]. Funct Mater Lett, 2013, 6(1):130-140. http://adsabs.harvard.edu/abs/2013FML.....650007Y [12] HUANG W, LI H, ZHU B, FENG Y. Selective hydrogenation of furfural to furfuryl alcohol over catalysts prepared via sonochemistry[J]. Ultrason Sonochem, 2007, 14(1):67-74. doi: 10.1016/j.ultsonch.2006.03.002 [13] DONG F, ZHU Y, ZHENG H. Cr-free Cu-catalysts for the selective hydrogenation of biomass-derived furfural to 2-methylfuran:The synergistic effect of metal and acid sites[J]. J Mol Catal A:Chem, 2015, 398:140-148. doi: 10.1016/j.molcata.2014.12.001 [14] NAKAGAWA Y, TAMURA M, TOMISHIGE K. Catalytic reduction of biomass-derived furanic compounds with hydrogen[J]. Acs Catal, 2013, 3(12):2655-2668. doi: 10.1021/cs400616p [15] 黄玉辉, 任国卿, 孙蛟, 王重庆, 陈晓蓉, 梅华.沉淀剂对CuZnAl催化剂糠醛气相加氢制糠醇选择性的影响[J].燃料化学学报, 2016, 44(6):726-731. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18851.shtmlHUANG Yu-hui, REN Guo-qing, SUN Jiao, WANG Chong-qing, CHEN Xiao-rong, MEI Hua. Effect of precipitant on the performance of CuZnAl catalysts in the gas phase selective hydrogenation of furfural to furfuryl alcohol[J]. J Fuel Chem Technol, 2016, 44(6):726-731. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18851.shtml [16] YANG J, ZHENG H Y, ZHU Y L. Effects of calcination temperature on performance of Cu-Zn-Al catalyst for synthesizing γ-butyrolactone and 2-methylfuran through the coupling of dehydrogenation and hydrogenation[J]. Catal Commun, 2004, 5(9):505-510. doi: 10.1016/j.catcom.2004.06.005 [17] 房德仁, 刘中民, 张慧敏.沉淀温度对CuO/ZnO/Al2O3系催化剂前驱体性质的影响[J].天然气化工:C1化学与化工, 2004, 29(4):28-32. http://www.cnki.com.cn/Article/CJFDTotal-TRQH200404007.htmFANG De-ren, LIU Zhong-ming, ZHANG Hui-ming. Influence of temperature on the properties of precursors of CuO/ZnO/Al2O3 catalysts[J]. Nat Gas Chem Ind, 2004, 29(4):28-32. http://www.cnki.com.cn/Article/CJFDTotal-TRQH200404007.htm [18] 姜广申, 胡云峰, 蔡俊.仲丁醇脱氢制甲乙酮的Cu-ZnO催化剂[J].化工进展, 2013, 32(2):352-358. http://www.cnki.com.cn/Article/CJFDTOTAL-HGJZ201302020.htmJIANG Guang-shen, HU Yun-feng, CAI Jun. Research of Cu-ZnO catalysts for sec-butanol dehydrogenation to methyl ethyl ketone[J]. Chem Ind Eng Prog, 2013, 32(2):352-358. http://www.cnki.com.cn/Article/CJFDTOTAL-HGJZ201302020.htm [19] CHOI Y, FUTAGAMI K, FUJITANI T. The role of ZnO in Cu/ZnO methanol synthesis catalysts morphology effect or active site model[J]. Appl Catal A:Gen, 2001, 208(1/2):163-167. [20] PARK S W, JOO O S, JUNG K D. Development of ZnO/Al2O3 catalyst for reverse-water-gas-shift reaction of CAMERE (carbon dioxide hydrogenation to form methanol via a reverse-water-gas-shift reaction) process[J]. Appl Catal A:Gen, 2001, 211(1):81-90. doi: 10.1016/S0926-860X(00)00840-1 [21] PEI T, LIU L, XU L. A novel glass fiber catalyst for the catalytic combustion of ethyl acetate[J]. Catal Commun, 2015, 74:19-23. https://www.researchgate.net/publication/283465666_A_novel_glass_fiber_catalyst_for_the_catalytic_combustion_of_ethyl_acetate -





 下载:
下载: