Preparation of tungstophosphoric acid intercalated MgAl layered double hydroxides with a tunable interlayer spacing and their catalytic esterification performance in the deacidification of crude oil
-
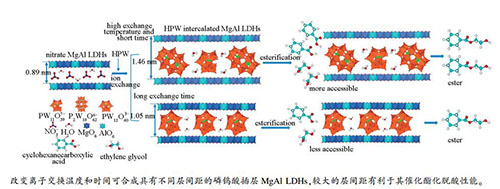
摘要: 通过改变离子交换温度和时间合成了具有不同层间距的磷钨酸(H3PW12O40,HPW)插层MgAl水滑石(LDHs),采用XRD、FT-IR、Raman、31P MAS NMR、ICP-AES和Hammett指示剂-正丁胺滴定法等表征其性质,并研究其对模型原油的催化酯化脱酸性能。高的离子交换温度有利于形成较大的层间距(d003约1.46 nm),较长的交换时间有利于形成较小的层间距(d003约1.05 nm)。不同的层间距源自HPW在层间不同的存在形式,P2W18O626-以C2轴倾斜于层板和PW11O397-以C2轴垂直于层板的方式排列于层间时,形成d003约1.46 nm的层间距;PW12O403-与层板发生嫁接,并以C2轴垂直于层板的方向排列于层间时,形成d003约1.05 nm的层间距。层间P2W18O626-和PW11O397-能产生更高比例的中强酸中心,同时大的层间距有利于反应物扩散进入层间与酸中心接触,能够提高LDHs的催化酯化脱酸性能。Abstract: This study demonstrates the synthesis of MgAl layered double hydroxides (LDHs) intercalated with tungstophosphoric acid (H3PW12O40, HPW) by an ion exchange method, and different interlayer spacings (d003) are obtained by adjusting the ion exchange temperature and time. The crystalline structures, molecular structures, atomic compositions, acidity, and specific surface areas of the LDH samples are rigorously characterized. A relatively high ion exchange temperature is demonstrated to be favorable for the formation of a large d003 value of around 1.46 nm, while a long exchange time is favorable for the formation of a small d003 value of around 1.05 nm. The different values of d003 are the result of different orientations of HPW anions within the interlayer space. Here, d003=1.46 nm is obtained when P2W18O626- and PW11O397- anions are arranged in the interlayer with their C2 axes respectively tilted toward and perpendicular to the LDH layer planes. In contrast, d003=1.05 nm is obtained when PW12O403- anions are grafted onto the LDH layers with their C2 axis perpendicular to the layer planes. Furthermore, the catalytic esterification performance of the samples is investigated for the deacidification of a model crude oil. Compared with PW12O403- anions, the presence of P2W18O626- and PW11O397- anions in the interlayer produce a higher proportion of sites with intermediate acidity that function as catalytic sites. Moreover, a large value of d003 facilitates the diffusion of reactants into the interlayer, which enhances their contact with the catalytic sites, and thereby increases the catalytic esterification performance of the LDHs in the deacidification of crude oil.
-
表 1 不同交换时间和温度合成的MgxAl-PW的d003及S6/S8值
Table 1 Interlayer spacing d003 and the S6/S8 ratios of the areas under the S6 and S8 diffraction peaks for MgxAl-PWy-z samples synthesized with different Mg/Al molar ratios x, ion exchange temperatures y, and ion exchange times z
Sample Temperature t/℃ Time t/h d003 (S6)/nm d003 (S8)/nm S6/S8a Mg2Al-PW25-1 25 1 1.44 1.07 0.04 Mg2Al-PW60-1 60 1 1.44 1.07 0.05 Mg2Al-PW80-1 80 1 1.44 1.07 0.11 Mg2Al-PW100-1 100 1 1.46 1.07 0.16 Mg2Al-PW100-3 100 3 1.44 1.06 0.08 Mg2Al-PW100-6 100 6 1.44 1.06 0.04 Mg2Al-PW100-12 100 12 - 1.05 0.00 Mg3Al-PW100-3 100 3 1.47 1.10 0.13 Mg3Al-PW100-6 100 6 1.46 1.10 0.09 Mg3Al-PW100-12 100 12 - 1.10 0.00 Mg4Al-PW100-3 100 3 1.49 1.10 0.12 Mg4Al-PW100-6 100 6 1.48 1.10 0.07 Mg4Al-PW100-12 100 12 - 1.10 0.00 a: the ratio of peak area of S6 to that of S8 表 2 不同层间距的Mg2Al-PW的分子式
Table 2 Extrapolated chemical formulae of Mg2Al-PWy-z samples with different S6/S8 values
Sample Mg/Al(molar ratio) Formula Mg2Al-PW100-1 1.86 Mg0.65Al0.35(OH)2(PW11O39)0.02(P2W18O62)0.03(PW12O40)0.01·mH2O Mg2Al-PW100-3 1.78 Mg0.64Al0.36(OH)2(PW11O39)0.01(P2W18O62)0.02(PW12O40)0.05·mH2O Mg2Al-PW100-6 1.73 Mg0.64Al0.37(OH)2(P2W18O62)0.01(PW12O40)0.10·mH2O Mg2Al-PW100-12 1.71 Mg0.63Al0.37(OH)2(PW12O40)0.12·mH2O 表 3 Mg2Al-NO3和Mg2Al-PW的酸性、比表面积和催化酯化脱酸性能
Table 3 Acidity, BET specific surface areas, and catalytic deacidification performances of Mg2Al-NO3 and Mg2Al-PWy-z catalysts
Sample Acid strength (H0) Amount of acidic sites /(mmol·g-1) Percentage of mid-strong acidic sites /% Specific surface area A /(m2·g-1) Deacidification ratio /% Mg2Al-NO3 0.8≤ H0 ≤7.2 0.164 - 10.3 33.9 0.8≤ H0 ≤7.2 0.601 Mg2Al-PW100-1 3.86≤ H0 ≤4.8 0.203 40.8 14.6 85.9 0.8≤ H0 ≤3.86 0.042 0.8≤ H0 ≤7.2 0.646 Mg2Al-PW100-3 3.86≤ H0 ≤4.8 0.198 36.7 17.1 82.4 0.8≤ H0 ≤3.86 0.039 0.8≤ H0 ≤7.2 0.654 Mg2Al-PW100-6 3.86≤ H0 ≤4.8 0.199 36.4 28.4 77.5 0.8≤ H0 ≤3.86 0.039 0.8≤ H0 ≤7.2 0.651 Mg2Al-PW100-12 3.86≤ H0 ≤4.8 0.197 36.1 39.2 74.2 0.8≤ H0 ≤3.86 0.038 表 4 不同Mg/Al物质的量比MgxAl-PW的催化酯化脱酸性能
Table 4 Catalytic deacidification performances of MgxAl-PWy-z samples with different values of x
Sample Deacidification ratio /% Sample Deacidification ratio /% Sample Deacidification ratio /% Mg3Al-PW100-3 73.8 Mg3Al-PW100-6 69.1 Mg3Al-PW100-12 67.7 Mg4Al-PW100-3 71.4 Mg4Al-PW100-6 67.4 Mg4Al-PW100-12 64.5 -
[1] SLAVCHEVA E, SHONE B, TURNBULL A. Review of naphthenic acid corrosion in oilrefining[J]. Brit Corros J, 1999, 34(2):125-131. doi: 10.1179/000705999101500761 [2] FU X, DAI Z, TIAN S, LONG J, WANG X. Catalytic decarboxylation of petroleum acids from high acid crude oils over solid acid catalysts[J]. Energy Fuels, 2008, 22(3):1923-1929. doi: 10.1021/ef7006547 [3] DASTJERDI Z, DUBÉ M A. Acid-catalyzed esterification of naphthenic acids[J]. Environ Prog Sustainable Energy, 2013, 32(2):406-410. doi: 10.1002/ep.11606 [4] WANG Y Z, LI J Y, SUN X Y, DUAN H L, SONG C M, ZHANG M M, LIU Y P. Removal of naphthenic acids from crude oils by fixed-bed catalytic esterification[J]. Fuel, 2014, 116:723-728. doi: 10.1016/j.fuel.2013.08.047 [5] CAVANI F, TRIFIRÒ F, VACCARI A. Hydrotalcite-type anionic clays:Preparation, properties and applications[J]. Catal Today, 1991, 11(2):173-301. http://d.old.wanfangdata.com.cn/NSTLQK/NSTL_QKJJ029328183/ [6] 段雪, 张法智.插层组装与功能材料[M].北京:化学工业出版社, 2007.DUAN Xue, ZHANG Fa-zhi. Intercalation Assembly and Functional Materials[M]. Beijing:Chemistry Industry Press, 2007. [7] 王恩波, 胡长文.多酸化学导论[M].北京:化学工业出版社, 1998.WANG En-bo, HU Chang-wen. Introduction of Polyacid Chemistry[M]. Beijing:Chemistry Industry Press, 1998. [8] WU Y, LIU X Y, LEI Y Q, QIU Y, WANG M L, WANG H. Synthesis and characterization of 12-tungstophosphoric acid intercalated layered double hydroxides and their application as esterification catalysts for deacidification of crude oil[J]. Appl Clay Sci, 2017, 150:34-41. doi: 10.1016/j.clay.2017.09.007 [9] POPE M T. Heteropoly and Isopoly Oxometalates[M]. Berlin:Springer, 1983. [10] 吴雁, 雷艳清, 刘新月, 邱月, 王豪. 12-磷钨酸插层MgAl水滑石的合成、表征及催化酯化原油脱酸性能[J].燃料化学学报, 2017, 45(9):1049-1055. doi: 10.3969/j.issn.0253-2409.2017.09.004WU Yan, LEI Yan-qing, LIU Xin-yue, QIU Yue, WANG Hao. Synthesis and characterization of 12-tungstophosphoric acid intercalated MgAl layered double hydroxides and their application as esterification catalysts for deacidification of crude oil[J]. J Fuel Chem Technol, 2017, 45(9):1049-1055. doi: 10.3969/j.issn.0253-2409.2017.09.004 [11] WANG H, DUAN W, WU Y, TANG Y, LI L. Synthesis of magnesium-aluminum layered double hydroxide intercalated with ethylene glycol by the aid of alkoxides[J]. Inorg Chim Acta, 2014, 418:163-170. doi: 10.1016/j.ica.2014.04.031 [12] JIA Y, FANG Y, ZHANG Y, MIRAS H N, SONG Y F. Classical Keggin intercalated into layered double hydroxides:Facile preparation and catalytic efficiency in Knoevenagel condensation reactions[J]. Chem-Eur J, 2015, 21(42):14862-14870. doi: 10.1002/chem.201501953 [13] MA J J, YANG M, CHEN Q, ZHANG S S, CHENG H, WANG S Y, LIU L, ZHANG C Y, TONG Z W, CHEN Z. Comparative study of Keggin-type polyoxometalate pillared layered double hydroxides via two synthetic routes:Characterization and catalytic behavior in green epoxidation of cyclohexene[J]. Appl Clay Sci, 2017, 150:210-216. doi: 10.1016/j.clay.2017.09.030 [14] ARCO M D, CARRIAZO D, GUTIÉRREZ S, MARTÍN C, RIVES V. Synthesis and characterization of new Mg2Al-paratungstate layered double hydroxides[J]. Inorg Chem, 2004, 43(1):375-384. doi: 10.1021/ic0347790 [15] WEIR M R, KYDD R A. Synthesis of heteropolyoxometalate-pillared Mg/Al, Mg/Ga, and Zn/Al layered double hydroxides via LDH-hydroxide precursors[J]. Inorg Chem, 1998, 37(21):5619-5624. doi: 10.1021/ic9805067 [16] YANG Q Z, SUN D J, ZHANG C G, WANG X J, ZHAO W A. Synthesis and characterization of polyoxyethylene sulfate intercalated Mg-Al-nitrate layered double hydroxide[J]. Langmuir, 2003, 19(14):5570-5574. doi: 10.1021/la034526j [17] BAJUK-BOGDANOVIĆ D, POPA A, USKOKOVIĆ-MARKOVIĆ S, HOLCLAJTNER-ANTUNOVIĆ I. Vibrational study of interaction between 12-tungstophosphoric acid and microporous/mesoporous supports[J]. Vib Spectrosc, 2017, 92:151-161. doi: 10.1016/j.vibspec.2017.06.007 [18] ZOU K, ZHANG H, DUAN X. Studies on the formation of 5-aminosalicylate intercalated Zn-Al layered double hydroxides as a function of Zn/Al molar ratios and synthesis routes[J]. Chem Eng Sci, 2007, 62(7):2022-2031. doi: 10.1016/j.ces.2006.12.041 [19] HOLCLAJTNER-ANTUNOVIĆ I, BAJUK-BOGDANOVIĆ D, POPA A, USKOKOVIĆ-MARKOVIĆ S. Spectroscopic identification of molecular species of 12-tungstophosphoric acid in methanol/water solutions[J]. Inorg Chim Acta, 2012, 383:26-32. doi: 10.1016/j.ica.2011.10.035 [20] LI Z S, JIA Y, WANG J, YANG P P, JING X Y, ZHANG M L. Synthesis and characterization of tungstophosphoric acid intercalated Ni/Al HTlc with magnetite[J]. J Mater Process Technol, 2009, 209(5):2613-2619. doi: 10.1016/j.jmatprotec.2008.06.005 [21] SMITH B J, PATRICK V A. Quantitative determination of aqueous dodecatungstophosphoric acid speciation by NMR spectroscopy[J]. Aust J Chem, 2004, 57(3):261-268. doi: 10.1071/CH02037 [22] CHEN Y, YAN D P, SONG Y F. Tris (hydroxymethyl) aminomethane modified layered double hydroxides greatly facilitate polyoxometalate intercalation[J]. Dalton Trans, 2014, 43(39):14570-14576. doi: 10.1039/C4DT01827C [23] BALLESTEROS M A, ULIBARRI M A, RIVES V, BARRIGA C. Optimum conditions for intercalation of lacunary tungstophosphate (Ⅴ) anions into layered Ni (Ⅱ)-Zn (Ⅱ) hydroxyacetate[J]. J Solid State Chem, 2008, 181(11):3086-3094. doi: 10.1016/j.jssc.2008.07.037 [24] XU L, HU C W, WANG E B. Advances in study of a new class of pillared layered microporous materials-polyoxometalate-type hydrotalcite-like catalysts[J]. J Nat Gas Chem, 1997, 6(2):155-168. [25] FUCHS V M, SOTO E L, BLANCO M N, PIZZIO L R. Direct modification with tungstophosphoric acid of mesoporous titania synthesized by urea-templated sol-gel reactions[J]. J Colloid Interface Sci, 2008, 327(2):403-411. doi: 10.1016/j.jcis.2008.08.045 [26] ZHU Z, TAIN R, RHODES C. A study of the decomposition behaviour of 12-tungstophosphate heteropolyacid in solution[J]. Can J Chem, 2003, 81(10):1044-1050. doi: 10.1139/v03-129 [27] ZHANG Y H, SU J X, WANG X P, PAN Q, QU W. Photocatalytic performance of polyoxometallate intercalated layered double hydroxide[C]//Materials Science Forum. Trans Tech Publications, 2011, 663: 187-190. [28] HIMENO S, TAKAMOTO M, UEDA T. Formation of α-and β-Keggin-type[PW12O40]3- complexes in aqueous media[J]. Bull Chem Soc Jpn, 2005, 78(8):1463-1468. doi: 10.1246/bcsj.78.1463 [29] LUO Q S, LI L, WANG Z X, DUAN X. Theoretical study of the layer-anion interactions in magnesium aluminum layered double hydroxides[J]. Chin J Inorg Chem, 2001, 17(6):835-842. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=wjhxxb200106012 [30] 胡长文, 刘彦勇, 贺庆林, 张继余, 王恩波.新型层柱催化剂ZnAl-XW11Z的酯化催化活性[J].催化学报, 1996, 17(3):237-240. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QK199600002923HU Chang-wen, LIU Yang-yong, HE Qing-lin, ZHANG Ji-yu, WANG En-bo. Studies on the esterification activity of a new type layer-pillared catalysts ZnAl-XW11Z[J]. Chin J Catal, 1996, 17(3):237-240. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QK199600002923 [31] JACOBS P A, MORTIER W J, UYTTERHOEVEN J B. Properties of zeolites in relation to their electronegativity:Acidity, carboniogenic activity and strength of interaction in transition metal complexes[J]. J Inorg Nucl Chem, 1978, 40(11):1919-1923. doi: 10.1016/0022-1902(78)80256-5 [32] AN Z, ZHANG W, SHI H, HE J. An effective heterogeneous L-proline catalyst for the asymmetric aldol reaction using anionic clays as intercalated support[J]. J Catal, 2006, 241(2):319-327. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=699f0b4878ffc1e2cfbea41ba04b075e -





 下载:
下载: