Pd/Al2O3 catalysts modified with Mg for catalytic combustion of methane: Effect of Mg/Al mole ratios on the supports and active PdOx formation
-
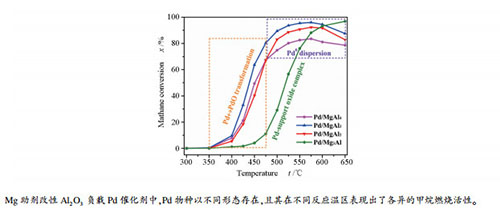
摘要: 本文研究了系列不同含量镁助剂改性的Pd/Al2O3催化剂的甲烷催化燃烧反应。研究表明,随着镁添加量的增加,载体由Al2O3转变为尖晶石型MgAl2O4,进一步增加Mg/Al物质的量比至3:1时,形成了Mg(Al)Ox固溶体;催化剂中活性相Pd物种以金属Pd,PdOx或Pd-载体复合氧化物形式存在,各物种的相对含量以及Pd↔PdO间的转化能力存在一定的差异。PdOx物种表现为具有较高的低温活性,而金属Pd和Pd-载体复合氧化物的高温活性较好。当Mg/Al物质的量比为1:3时,催化剂的Pd↔PdO转化能力最强,表现出了最高的甲烷催化燃烧反应活性。Abstract: Pd/Al2O3 catalysts modified by different amount of magnesium were fabricated for catalytic combustion of methane (CCM). After the introduction of different amount of magnesium, Al2O3, MgAl2O4-like mixed oxide and Mg(Al)Ox solid solution were formed. Owing to the formation of distinguished supports, the supported Pd species, i.e. metallic Pd, PdOx and support-Pd oxide complex were formed, and they were quite different in relative content and Pd↔PdO transformation ability. It was found that PdOx was active at low temperature, while metallic Pd particles and support-Pd oxide complex were active at high reaction temperature. The one with Mg/Al mole ratio of 1:3 was the most easily in Pd↔PdO transformation, demonstrating the best catalytic activity towards CCM reaction.
-
Key words:
- methane catalytic combustion /
- Pd-based catalyst /
- MgAl2O4 /
- Mg(Al)Ox
-
Table 1 Physical-chemical properties of the Pd/MgxAlyO catalysts with different Mg/Al mole ratios
Sample Pd loadinga/% Mg/Ala (at.) PdO crystal size b/nm ABETc/ (m2·g-1) thystersisd/ ℃ H2 consumptione/ (μmol·g-1) H2 consumption or PdOxf/ (μmol·g-1) Pd surface areag/ (m2·g-1) Pd/Al2O3 0.96 0 18.3 168 136 11 4.0 - Pd/MgAl4 0.97 1:3.8 15.1 135 144 82 68 46.5 Pd/MgAl3 0.96 1:3.1 15.3 126 119 83 62 56.4 Pd/MgAl2 1.04 1:1.9 13.8 122 154 72 58 67.2 Pd/Mg3Al 0.97 3.2:1 11.9 180 280 9.0 - - a: determined by the ICP-OES analysis;
b: estimated from PdO (101) diffraction peak at ca.39.5° by Scherrer equation;
c: BET surface area calculated from N2 physisorption isotherm;
d: temperature hysteresis (thystersis) between the heating and cooling cycles from TPO profiles in Figure 4, for example, in Pd/Al2O3 catalyst the decomposition of PdOx starts at 710 ℃ during the heating process, while the oxidation of Pd starts at 574 ℃ during the cooling process, so thystersis=(710-574) ℃ =136 ℃;
e: H2 consumption calculated from H2-TPR profiles (sum of peak α and β);
f: H2 consumption calculated from H2-TPR profiles (subtracting H2 consumption of peak α and β to that of peak γ);
g: Pd surface area derived from the H2-O2 titration experiment -
[1] HAYES R E. Catalytic solutions for fugitive methane emissions in the oil and gas sector[J]. Chem Eng Sci, 2004, 59(19):4073-4080. doi: 10.1016/j.ces.2004.04.038 [2] HONG E, KIM C, LIM D H, CHO H J, SHIN C H. Catalytic methane combustion over Pd/ZrO2 catalysts:Effects of crystalline structure and textural properties[J]. Appl Catal B:Environ, 2018, 232:544-552. doi: 10.1016/j.apcatb.2018.03.101 [3] GLUHOI A C, NIEUWENHUYS B E. Catalytic oxidation of saturated hydrocarbons on multicomponent Au/Al2O3 catalysts:Effect of various promoters[J]. Catal Today, 2007, 119(1):305-310. http://www.sciencedirect.com/science/article/pii/S0920586106005682 [4] TOSO A, COLUSSI S, PADIGAPATY S, DE LEITENBURG C, TROVARELLI A. High stability and activity of solution combustion synthesized Pd-based catalysts for methane combustion in presence of water[J]. Appl Catal B:Environ, 2018, 230:237-245. doi: 10.1016/j.apcatb.2018.02.049 [5] CHEN J H, ARANDIYAN H, GAO X, LI J H. Recent advances in catalysts for methane combustion[J]. Catal Surv Asia, 2015, 19(3):140-171. doi: 10.1007/s10563-015-9191-5 [6] SU Y Q, FILOT I A W, LIU J X, HENSENG E J M. Stable Pd-doped ceria structures for CH4 activation and CO oxidation[J]. ACS Catal, 2018, 8(1):75-80. doi: 10.1021/acscatal.7b03295 [7] FRIBERG I, SADOKHINA N, OLSSON L. Complete methane oxidation over Ba modified Pd/Al2O3:The effect of water vapor[J]. Appl Catal B:Environ, 2018, 231:242-250. doi: 10.1016/j.apcatb.2018.03.003 [8] DAI Q G, ZHU Q, LOU Y, WANG X Y. Role of Bronsted acid site during catalytic combustion of methane over PdO/ZSM-5:Dominant or negligible?[J]. J Catal, 2018, 357:29-40. doi: 10.1016/j.jcat.2017.09.022 [9] CHIN Y H, GARCÍA-DIÉGUEZ M, IGLESIA E. Dynamics and thermodynamics of Pd-PdO phase transitions:Effects of Pd cluster size and kinetic implications for catalytic methane combustion[J]. J Phys Chem C, 2016, 120(3):1446-1460. doi: 10.1021/acs.jpcc.5b06677 [10] CHIN Y H, BUDA C, NEUROCK M, IGLESIA E. Consequences of metal-oxide interconversion for C-H bond activation during CH4 reactions on pd catalysts[J]. J Am Chem Soc, 2013, 135(41):15425-15442. doi: 10.1021/ja405004m [11] MONTEIRO R S, ZEMLYANOV D, STOREY J M, RIBEIRO F H. Turnover rate and reaction orders for the complete oxidation of methane on a palladium foil in excess dioxygen[J]. J Catal, 2001, 199(2):291-301. doi: 10.1006/jcat.2001.3176 [12] WILLIS J J, GALLO A, SOKARAS D, ALJAMA H, NOWAK S H, GOODMAN ED, WU L, TASSONE CJ, JARAMILLO T F, ABILD-PEDERSEN F, CARGNELLO M. Systematic structure-property relationship studies in palladium-catalyzed methane complete combustion[J]. ACS Catal, 2017, 7(11):7810-7821. doi: 10.1021/acscatal.7b02414 [13] MURATA K, MAHARA Y, OHYAMA J, YAMAMOTO Y, ARAI S, SATSUMA A. The metal-support interaction concerning the particle size effect of Pd/Al2O3 on methane combustion[J]. Angew Chem Int Ed, 2017, 56(50):15993-15997. doi: 10.1002/anie.201709124 [14] HUANG F J, CHEN J J, HU W, LI G X, WU Y, YUAN S D, ZHONG L, CHEN Y Q. Pd or PdO:Catalytic active site of methane oxidation operated close to stoichiometric air-to-fuel for natural gas vehicles[J]. Appl Catal B:Environ, 2017, 219:73-81. doi: 10.1016/j.apcatb.2017.07.037 [15] FARRAUTO R J, LAMPERT J K, HOBSON M C, WATERMAN E M. Thermal decomposition and reformation of PdO catalysts; support effects[J]. Appl Catal B:Environ, 1995, 6(3):263-270. doi: 10.1016/0926-3373(95)00015-1 [16] NILSSON J, CARLSSON P-A, FOULADVAND S, MARTIN N M, GUSTAFSON J, NEWTON M A, LUNDGREN E, GRÖNBECK H, SKOGLUNDH M. Chemistry of supported palladium nanoparticles during methane oxidation[J]. ACS Catal, 2015, 5(4):2481-2489. doi: 10.1021/cs502036d [17] ONN T M, ARROYO-RAMIREZ L, MONAI M, OH T S, TALATI M, FORNASIERO P, GORTE R J, KHADER M M. Modification of Pd/CeO2 catalyst by atomic layer deposition of ZrO2[J]. Appl Catal B:Environ, 2016, 197:280-285. doi: 10.1016/j.apcatb.2015.12.028 [18] BEN SAID I, SADOUKI K, MASSE S, CORADIN T, SMIRI LS, FESSI S. Advanced Pd/CexZr(l-x)O2/MCM-41 catalysts for methane combustion:Effect of the zirconium and cerium loadings[J]. Microporous Mesoporous Mater, 2018, 260:93-101. doi: 10.1016/j.micromeso.2016.10.044 [19] THEVENIN P O, ALCALDE A, PETTERSSON L J, JÄRÅS S G, FIERRO J L G. Catalytic combustion of methane over cerium-doped palladium catalysts[J]. J Catal, 2003, 215(1):78-86. doi: 10.1016/S0021-9517(02)00146-X [20] COLUSSI S, TROVARELLI A, VESSELLI E, BARALDI A, COMELLI G, GROPPI G, LLORCA J. Structure and morphology of Pd/Al2O3 and Pd/CeO2/Al2O3 combustion catalysts in Pd-PdO transformation hysteresis[J]. Appl Catal A:Gen, 2010, 390(1):1-10. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=29f2bc79b52ef7402ea5107dca7113d8 [21] SHI C, ZHANG P. Role of MgO over γ-Al2O3-supported Pd catalysts for carbon dioxide reforming of methane[J]. Appl Catal B:Environ, 2015, 170-171:43-52. doi: 10.1016/j.apcatb.2015.01.034 [22] YANG L F, SHI C K, HE X E, CAI J X. Catalytic combustion of methane over PdO supported on Mg-modified alumina[J]. Appl Catal B:Environ, 2002, 38(2):117-125. doi: 10.1016/S0926-3373(02)00034-6 [23] FENG J T, WANG H Y, EVANS D G, DUAN X, LI D Q. Catalytic hydrogenation of ethylanthraquinone over highly dispersed eggshell Pd/SiO2-Al2O3 spherical catalysts[J]. Appl Catal A:Gen, 2010, 382(2):240-245. doi: 10.1016/j.apcata.2010.04.052 [24] LI D, LI R, LU M, LIN X, ZHAN Y, JIANG L. Carbon dioxide reforming of methane over Ru catalysts supported on Mg-Al oxides:A highly dispersed and stable Ru/Mg(Al)O catalyst[J]. Appl Catal B:Environ, 2017, 200:566-577. doi: 10.1016/j.apcatb.2016.07.050 [25] LI D, LU M, CAI Y, CAO Y, ZHAN Y, JIANG L. Synthesis of high surface area MgAl2O4 spinel as catalyst support via layered double hydroxides-containing precursor[J]. Appl Clay Sci, 2016, 132/133:243-250. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=d5211dc927ad4ca9e98415a618c52196 [26] OHI T, MIYATA T, LI D, SHISHIDO T, KAWABATA T, SANO T, TAKEHIRA K. Sustainability of Ni loaded Mg-Al mixed oxide catalyst in daily startup and shutdown operations of CH4 steam reforming[J]. Appl Catal A:Gen, 2006, 308:194-203. doi: 10.1016/j.apcata.2006.04.025 [27] LIN X, LI R, LU M, CHEN C, LI D, ZHAN Y, JIANG L. Carbon dioxide reforming of methane over Ni catalysts prepared from Ni-Mg-Al layered double hydroxides:Influence of Ni loadings[J]. Fuel, 2015, 162:271-280. doi: 10.1016/j.fuel.2015.09.021 [28] CAVANI F, TRIFIRÒ F, VACCARI A. Hydrotalcite-type anionic clays:Preparation, properties and applications[J]. Catal Today, 1991, 11(2):173-301. doi: 10.1016/0920-5861(91)80068-K [29] SABERI A, GOLESTANI-FARD F, SARPOOLAKY H, WILLERT-PORADA M, GERDES T, SIMON R. Chemical synthesis of nanocrystalline magnesium aluminate spinel via nitrate-citrate combustion route[J]. J Alloy Compd, 2008, 462(1):142-146. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=459f3f42065bd64abd67ffce88a7755c [30] VILLA A, GAIASSI A, ROSSETTI I, BIANCHI C L, VAN BENTHEM K, VEITH G M, PRATI L. Au on MgAl2O4 spinels:The effect of support surface properties in glycerol oxidation[J]. J Catal, 2010, 275(1):108-116. doi: 10.1016/j.jcat.2010.07.022 [31] GROPPI G, CRISTIANI C, LIETTI L, FORZATTI P. Study of PdO/Pd transformation over alumina supported catalysts for natural gas combustion[J]. Stud Surf Sci Catal, 2000, 130:3801-3806. doi: 10.1016/S0167-2991(00)80615-1 [32] FARRAUTO R J, HOBSON M C, KENNELLY T, WATERMAN E M. Catalytic chemistry of supported palladium for combustion of methane[J]. Appl Catal A:Gen, 1992, 81(2):227-237. doi: 10.1016/0926-860X(92)80095-T [33] MCCARTY J G. Kinetics of PdO combustion catalysis[J]. Catal Today, 1995, 26(3):283-293. doi: 10.1016-0920-5861(95)00150-7/ [34] SCHWARTZ W R, PFEFFERLE L D. Combustion of methane over palladium-based catalysts:Support Interactions[J]. J Phys Chem C, 2012, 116(15):8571-8578. doi: 10.1021/jp2119668 -




 下载:
下载: