Preparation of MnO2/PoPD@PPS functional composites for low-temperature NO reduction with NH3
-
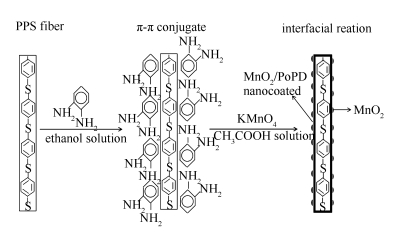
摘要: 在聚苯硫醚(PPS)滤料表面包覆一层二氧化锰/聚邻苯二胺(PoPD)复合物。利用π-π共轭效应,将邻苯二胺(OPD)单体均匀吸附在PPS纤维表面,然后通过高锰酸钾溶液使邻苯二胺氧化聚合,在纤维表面原位生成聚邻苯二胺包覆层,同时高锰酸钾被还原成MnO2催化剂,插入到聚邻苯二胺基体中。通过原位聚合生成的MnO2/PoPD复合物与PPS滤料间有很强的黏结性,使得催化剂和滤料能牢固地结合在一起。该复合滤料制备方法简单,实验条件温和,对滤料本身性能没有损伤,通过FESEM、XPS、XRD、FT-IR、脱硝活性测试等对其结构和性能进行了研究。脱硝测试结果表明,KMnO4/PPS质量比为1:1时,复合滤料在80-180 ℃下脱硝率可达36%-94%,10 h的催化剂稳定性测试中,其脱硝率在160 ℃下仍保持在88%;Mn 2p的XPS谱图证实复合滤料上催化剂为MnO2;复合滤料的XRD谱图表明MnO2为非晶结构;从FESEM照片可以看出,MnO2催化剂在PPS滤料上分散均匀。Abstract: A layer of manganese dioxide/poly (p-phenylenediamine) (PoPD) complex was coated on the surface of polyphenylene sulfide (PPS). First, the o-phenylenediamine (OPD) monomer was uniformly adsorbed on the surface of the PPS fiber by the effect of π-π conjugation. Then, the o-phenylenediamine was oxidized by the potassium permanganate solution to produce poly (p-phenylenediamine) coat, while the potassium permanganate was reduced to MnO2 catalyst and inserted into the poly (o-phenylenediamine) matrix. The MnO2 catalyst was firmly bonded with the PPS filter because the MnO2/PoPD complex formed by in-situ polymerization exhibited a strong bond with the PPS filter. The preparation method of MnO2/PoPD@PPS composite filter was simple. Due to the mild experimental conditions, the performance of the PPS filter media was not damaged. The structure and properties of MnO2/PoPD@PPS composite filter were studied in detail by FESEM, XPS, XRD, FT-IR and denitrification test. The results of denitration test show that the denitrification rate of MnO2/PoPD@PPS composite filter increases with the increase of KMnO4/PPS mass ratio. The optimum denitrification rate is 36%-94% at 80-180℃ with the KMnO4/PPS mass ratio of 1:1, and it is 88% at 160℃ after 10 h catalyst stability test. The XPS spectrum of Mn 2p proves that the catalyst on the composite filter is MnO2 that possesses amorphous structure observed from XRD patterns. It can be observed from the FESEM diagram that the dispersion of the MnO2 catalyst on the PPS filter is uniform.
-
Key words:
- polyphenylene sulfide (PPS) /
- MnO2 /
- in-situ polymerization /
- NH3-SCR
-
表 1 1.0 MnO2/PoPD@PPS复合滤料的N2选择性
Table 1 N2 selectivity of the 1.0 MnO2/PoPD@PPS
Temperature t/℃ Selectivity s/% N2 NOx N2O 80 - - - 100 - - - 120 81 1 18 140 81 1 18 160 81 1 18 180 81 1 18 reaction conditions: 5×10-4 NO, 5×10-4 NH3, 5% O2 and balanced by N2, about 120 000 cm3/(g·h) of GHSV -
[1] SHEN Bo-xiong, GUO Bin-bin, SHI Zhan-liang, WU Chun-fei, LIANG Cai. Low temperature SCR of NO in flue gas on CeO2/ACF[J]. J Fuel Chem Technol, 2007, 35(1): 125-128. http://en.cnki.com.cn/Article_en/CJFDTOTAL-RLHX200701026.htm [2] ZHANG Xiao-peng, SHEN Bo-xiong. Selective catalytic reduction of NO with NH3 over Mn-based catalysts at low temperature[J]. J Fuel Chem Technol, 2013, 41(1): 123-128. http://en.cnki.com.cn/Article_en/CJFDTOTAL-RLHX201301020.htm [3] SHEN Mei-qing, LI Chen-xu, WANG Jian-qiang, XU Li-li, WANG Wu-lin, WANG Jun. New insight into the promotion effect of Cu doped V2O5/WO3-TiO2 for low temperature NH3-SCR performance[J]. Rsc Adv, 2015, 5(44): 35155-35165. doi: 10.1039/C5RA04940G [4] LIAN Zhi-hua, LIU Fu-dong, HE Hong, LIU Kuo. Nb-doped VOx/CeO2 catalyst for NH3-SCR of NOx at low temperatures[J]. Rsc Adv, 2015, 5(47): 37675-37681. doi: 10.1039/C5RA02752G [5] LIU Qing, ZHENG Yu-ying, WANG Xie. Research on de-NO by low-temperature SCR based on MnOx-CeO2/PPSN[J]. J Fuel Chem Technol, 2012, 4: 452-455. http://www.sciencedirect.com/science/article/pii/S1872581312600206 [6] QIU Yun-shun, SHEN Yue-song, YANG Bo, SHEN Shu-bao, ZHU She-min. Study of Influencing Factors on Low-temperature Catalytic Performance of Mn-Ce-Ni-Ox/PPS Filter for NH3-SCR of NO[J]. Coal Technol, 2015, 2: 316-318. [7] YANG Bo, SHEN Yue-song, QIU Yun-shun, ZENG Yan-wei, SHEN Shu-bao, ZHU She-min. Influencing factors on low-temperature deNOx performance of Mn-La-Ce-Ni-Ox/P84[J]. Chinese J Environmental Engine, 2016, 10(11): 6583-6587. [8] CHEN Xiao-jun, ZHANG Qi, QIAN Chun-hua, HAO Ning, LIN Xub, CHENG Yaoa. Electrochemical aptasensor for mucin 1 based on dual signal amplification of poly(o-phenylenediamine) carrier and functionalized carbon nanotubes tracing tag[J]. Biosen Bioelectron, 2015, 64(22): 485. http://www.sciencedirect.com/science/article/pii/S0956566314007398 [9] YUAN C, LIU X, JIA M, LUO Z, YAO J. Facile preparation of N-and O-doped hollow carbon spheres derived from poly(o-phenylenediamine) for supercapacitors[J]. Chem Mater, 2015, 3(7): 3409-3415. doi: 10.1039/C4TA06411A [10] ZHANG Y, ZHENG Y, WANG X, LU X. Preparation of Mn-FeOx /CNTs catalysts by redox co-precipitation and application in low-temperature NO reduction with NH3[J]. J Catal Commun, 2015, 62: 57-61. doi: 10.1016/j.catcom.2014.12.023 [11] QIU Bin, XU Cui-xia, SUN De-zhi, WANG Qiang, GU Hong-bo, ZHANG Xin, WEEKS B L, HOPPER J, HOT C, GUO Zhan-hu, WEI Su-ying. Polyaniline coating with various substrates for hexavalent chromium removal[J]. Appl Surf Sci, 2015, 334: 7-14. doi: 10.1016/j.apsusc.2014.07.039 [12] YU Jun, SI Zhi-chun, CHEN Lei, WU Xiao-dong, WENG Duan. Selective catalytic reduction of NOx by ammonia over phosphate-containing Ce0.75Zr0.25O2 solids[J]. Appl Catal B: Environmental, 2015, 163: 223-232. http://www.sciencedirect.com/science/article/pii/S0926337314004779 [13] WANG Ji-hui, DONG Xue-song, WANG Yu-jie, LI Yong-dan. Effect of the calcination temperature on the performance of a CeMoOx catalyst in the selective catalytic reduction of NOx with ammonia[J]. Catal Today, 2015, 245: 10-15. doi: 10.1016/j.cattod.2014.07.035 [14] HUANG Xue-hui, NIU Peng-ju, SHANG Xiao-hui. Low temperature molten salt synthesis of porous La1-xSrxMn0.8Fe0.2O3 (0≤x≤0.6) microspheres with high catalytic activity for CO oxidation[J]. Chinese J Catal, 2016, 37(8): 1431-1439. doi: 10.1016/S1872-2067(16)62502-0 [15] SHEN Yu, WANG Lian-feng, WU Yan-bo, LI Xin-yong, ZHAO Qi-dong, HOU Yang, TENG Wei. Facile solvothermal synthesis of MnFe2O4, hollow nanospheres and their photocatalytic degradation of benzene investigated by in situ FT-IR[J]. Catal Commun, 2015, 68: 11-14. doi: 10.1016/j.catcom.2015.04.025 [16] LI Na, ZHU Xiao-hong, ZHANG Cai-yun, LAI Liu-qin. Fabrication of PANI-coated honeycomb-like MnO2 nanosphereswith enhanced electrochemical performance for energy storage[J]. Electro Acta, 2015, 180: 977-982. doi: 10.1016/j.electacta.2015.09.056 [17] ZHANG Yan-bing, ZHENG Yu-ying, WANG Xie, LU Xiu-lian. Preparation of Mn-FeOx/CNTs catalysts by redox co-precipitation and application in low-temperature NO reduction with NH3[J]. Catal Communs, 2015, 62: 57-61. doi: 10.1016/j.catcom.2014.12.023 -





 下载:
下载: