Migration and transformation characteristics of zigzag char-N in lean oxygen environment
-
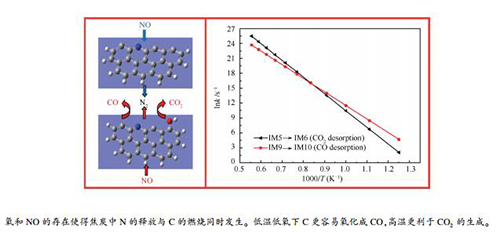
摘要: 采用量子化学方法探究了还原区高浓度NO存在下zigzag结构焦炭氮中N的迁移转化规律,并通过构建含羟基焦炭N模型,从分子层面对氧存在下焦炭N的转化特性进行了系统的理论计算。结果表明,还原区NO的存在会与焦炭中的N结合为N2释放;并且氧的存在增强了焦炭表面化学活性,进一步促进了焦炭中N的析出。还原区氧和NO的共存使得焦炭中N的释放与C的燃烧同时发生,表现为NO与焦炭中N结合为N2的同时,伴随有氧将焦炭中C氧化成CO2或CO。动力学计算C燃烧产物的限速步速率常数发现,低温低氧条件下C更容易氧化生成CO;随着温度的升高,CO2生成速率明显增大,高温更利于CO2的生成。Abstract: The migration and transformation of N in zigzag char-N with the presence of high concentration NO in the reduction zone is investigated by quantum chemistry method. Transformation characteristics of N in lean oxygen environment are systematically calculated from the molecular level by constructing a char-N model containing a hydroxyl group. The results show that NO in the reduction zone can combine with N in the char to form N2; and the presence of oxygen enhances the char chemical activity and further promotes the release of N in the char. The co-existence of oxygen and NO in the reduction zone makes the release of N and the combustion of C occur simultaneously, which is manifested by NO and N in the char combining to form N2, and at the same time oxygen and C in the char formation CO2 or CO. The kinetic calculations of the rate-limiting step rate constants of the C combustion products show that C is easily oxidized to CO under low temperature and lean oxygen conditions, and with the temperature rise the CO2 generation rate increases significantly and the high temperature is conducive to CO2 formation.
-
Key words:
- char-N /
- NO /
- hydroxyl group /
- CO /
- CO2
-
表 1 NBO计算的一些重要原子的自然密度
Table 1 Natural population of some important atoms calculated by NBO
Atom Species Charge Valence N3 IM4 -0.11611 2.60384 IM5 -0.06121 2.54506 C7 IM4 0.08531 1.90433 IM5 0.17767 1.81130 N8 IM4 0.12140 2.35500 IM5 0.08398 2.4017] O9 IM4 -0.19029 3.18171 IM5 -0.20992 3.20406 -
[1] FAN W D, LI Y, GUO Q H, CHEN C, WANG Y. Coal-nitrogen release and NOx evolution in the oxidant-staged combustion of coal[J]. Energy, 2017, 125:417-426. doi: 10.1016/j.energy.2017.02.130 [2] CODA B, KLUGER F, FORTSCH D, SPLIETHOFF H, HEIN K R G. Coal-nitrogen release and NOx evolution in air-staged combustion[J]. Energy Fuels, 1998;12:1322-1327. doi: 10.1021/ef980097z [3] STADLER H, CHRIST D, HABERMEHL M, HEIL P, KELLERMANN A, OHLIGER A, TOPOROV D, KNEER R. Experimental investigation of NOx emissions in oxycoal combustion[J]. Fuel, 2011, 90(4):1604-1611. https://www.sciencedirect.com/science/article/pii/S0959652618319899 [4] WANG J, FAN W, LI Y, XIAO M, WANG K, REN P. The effect of air staged combustion on NOx emission in dried lignite combustion[J]. Energy, 2012, 37(1):725-736. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=91c6de7648651ae3f3c528774b81ed2c [5] FAN W, LIN Z, KUANG J, LI Y. Impact of air staging along furnace height on NOx emissions from pulverized coal combustion[J]. Fuel Process Technol, 2010, 91(6):625-634. doi: 10.1016/j.fuproc.2010.01.009 [6] ZHANG X X, ZHOU Z J, ZHOU J H, LIU J Z, CEN K F. Density functional study of NO desorption from oxidation of nitrogen containing char by O2[J]. Combust Sci Technol, 2012, 184(4):445-455. doi: 10.1080/00102202.2011.648031 [7] SENDT K, HAYNES B S. Density functional study of the chemisorption of O2 on the armchair surface of graphite[J]. P Combust Inst, 2005, 30(2):2141-2149. doi: 10.1016/j.proci.2004.08.064 [8] SENDT K, HAYNES B S. Density functional study of the chemisorption of O2 on the zigzag surface of graphite[J]. Combust Flame, 2005, 143(4):629-643. http://www.sciencedirect.com/science/article/pii/S001021800500249X [9] CHEN P, GU M Y, CHEN X, CHEN J C. Study of the reaction mechanism of oxygen to heterogeneous reduction of NO by char[J]. Fuel, 2019, 236:1213-1225. doi: 10.1016/j.fuel.2018.09.094 [10] 高正阳, 杨维结, 阎维平.煤焦催化HCN还原NO的反应机理[J].燃料化学学报, 2017, 45(9):1043-1048. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rlhxxb201709003GAO Zheng-yang, YANG Wei-jie, YAN Wei-ping. Reaction mechanism of NO reduction with HCN catalyzed by char[J]. J Fuel Chem Technol, 2017, 45(9):1043-1048. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rlhxxb201709003 [11] ZHANG X X, XIE M, WU H X, LV X X, ZHOU Z J. DFT study of the effect of Ca on NO heterogeneous reduction by char[J]. Fuel, 2020, 265:116995. doi: 10.1016/j.fuel.2019.116995 [12] HOSKINS B L, MILKE J A. Differences in measurement methods for travel distance and area for estimates of occupant speed on stairs[J]. Fire Safety J, 2012, 48:49-57. doi: 10.1016/j.firesaf.2011.12.009 [13] ZHUANG X G, YANG Y S, YANG D P, JI Y J, TANG Z Y. Effect of surface functional groups on the properties of activated carbon[J]. Batt Bimon, 2003, 33(4):199-202. [14] QI X Y, XUE H B, XIN H H, WEI C X. Reaction pathways of hydroxyl groups during coal spontaneous combustion[J]. Can J Chem, 2016, 94:494-500. doi: 10.1139/cjc-2015-0605 [15] ZHANG H, JIANG X M, LIU J X, SHEN J. Application of density functional theory to the nitric oxide heterogeneous reduction mechanism in the presence of hydroxyl and carbonyl groups[J]. Energy Conver Manage, 2014, 83:167-176. doi: 10.1016/j.enconman.2014.03.067 [16] 肖萌, 王俊超, 李宇, 范卫东.高温下煤焦表面含氧官能团对NO-煤焦还原反应的影响[J].热能动力工程, 2012, 27(2):227-231. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rndlgc201202017XIAO Meng, WANG Jun-chao, LI Yu, FAN Wei-dong. Effect of oxygen-containing functional groups on no char reduction at high temperature[J]. J Eng Therm Pow, 2012, 27(2):227-231. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rndlgc201202017 [17] 陈萍, 顾明言, 汪嘉伦, 卢坤, 林郁郁.含氮煤焦还原NO反应路径研究[J].燃料化学学报, 2019, 47(3):279-286. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rlhxxb201903004CHEN Ping, GU Ming-yan, WANG Jia-lun, LU Kun, LIN Yu-yu. Reaction pathways for the reduction of NO by nitrogen-containing char[J]. J Fuel Chem Technol, 2019, 47(3):279-286. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rlhxxb201903004 [18] MONTOYA A, TRUONG T N, SAROFIM A F. Application of density functional theory to the study of the reaction of NO with char-bound nitrogen during combustion[J]. J Phys Chem A, 2000, 104(36):8409-8417. doi: 10.1021/jp001045p [19] PHAM B Q, NGUYEN V H, TRUONG T N. Size dependence of graphene chemistry:A computational study on CO desorption reaction[J]. Carbon, 2016, 101:16-21. doi: 10.1016/j.carbon.2016.01.028 [20] CALDERÓN L A, CHAMORRO E, ESPINAL J F. Mechanisms for homogeneous and heterogeneous formation of methane during the carbon-hydrogen reaction over zigzag edge sites[J]. Carbon, 2016, 102:390-402. doi: 10.1016/j.carbon.2016.02.052 [21] ESPINAL J F, TRUONG T N, MONDRAGÓ N F. Mechanisms of NH3 formation during the reaction of H2 with nitrogen containing carbonaceous materials[J]. Carbon, 2007, 45(11):2273-2279. doi: 10.1016/j.carbon.2007.06.011 [22] PHAM B Q, TRUONG T N. Electronic spin transitions in finite-size graphene[J]. Chem Phy Lett, 2012, 535:75-79. doi: 10.1016/j.cplett.2012.03.041 [23] FRISCH M J, TRUCKS G W, SCHLEGEL H B, et al. Revision B.01[CP]. Wallingford CT: Gaussian Inc.; 2010. [24] LAIDLER K J, KING M C. The development of transition-state theory[J]. J Phys Chem, 1983, 87:2657-2664. doi: 10.1021/j100238a002 [25] WIGNER E. Concerning the excess of potential barriers in chemical reactions[J]. Z Phys Chem B:Chem E, 1932, 19:203-216. [26] YAN W X, LI S G, FAN S G, DENG S. Effect of surface carbon-oxygen complexes during NO reduction by coal char[J]. Fuel, 2017, 204:40-46. doi: 10.1016/j.fuel.2017.05.045 -





 下载:
下载: