Research progress of the influence of alkali metals and alkaline earth metals on coal thermal chemical conversion
-
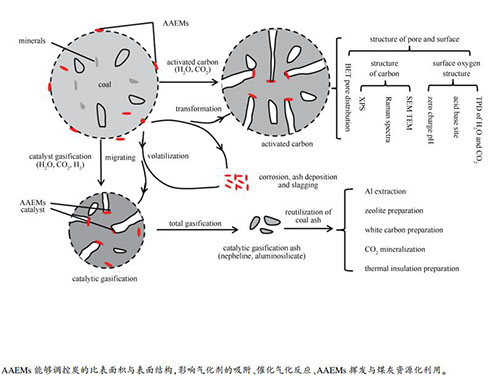
摘要: 对比阐述了AAEMs在煤炭热转化过程的影响与作用,论述了催化气化中炭结构转变、碱金属形态变化、催化剂失活等过程。AAEMs是催化气化的催化剂,是活性炭制备过程的造孔剂,也是高AAEMs煤利用过程的有害组分,同样也是煤灰提铝过程的焙烧活化剂。AAEMs与炭相互作用影响炭的表面结构,进而影响气化剂在炭表面的吸附与反应,而AAEMs与炭的相互作用也会影响AAEMs的挥发与释放,耦合催化气化与煤灰资源化利用可以有效降低催化剂回收成本。通过对比认识AAEMs在煤炭热转化中的影响与作用,以期为AAEMs作用下煤炭热转化过程提供新思路与方法。Abstract: In order to comprehensively understand the catalytic gasification process, the influence of AAEMs during coal thermal chemical conversion was compared and explained. The main issues related to the catalytic gasification, such as the transformation of carbon structure, the conversion of alkali metals, and the deactivation of catalysts were discussed. AAEMs can be used as the catalysts for catalytic gasification, the pore making additives for activated carbon preparation, the activating agents in the process of aluminum extraction from coal ash by roasting, but also be regarded as hazard elements for high AAEMs coal utilization. The interaction between AAEMs and carbon can affect the surface structure of carbon to favor the adsorption and reaction of gasification agent on the carbon surface. As the same time, the volatilization and release of AAEMs can not be escaped, but the catalyst recovery can be effectively promoted by coupling the catalytic gasification and the coal ash resource utilization. By understanding the effect of AAEMs on coal thermal chemical conversion, we can propose novel ideas and innovation techniques for coal thermal chemical conversion with AAEMs addition.
-
图 6 K2CO3催化气化过程中间产物转化[18]
Figure 6 Transformation of intermediate during K2CO3 catalytic gasification
图 8 炭体积扩散机理示意图[25]
Figure 8 Diagrammatic sketch of carbon bulk diffusion
图 9 煤焦气化过程Na形成的沟槽结构[29]
Figure 9 Formation of channeling structure by Na during catalytic gasification
-
[1] JIAO W, WANG Z, ZHOU X, MEI Y, FENG R, LIU T, DING L, HUANG J, FANG Y. Catalytic steam gasification of sawdust char on K-based composite catalyst at high pressure and low temperature[J]. Chem Eng Sci, 2019, 205:341-349. doi: 10.1016/j.ces.2019.05.009 [2] LI G, LIU Z, LI J, FANG Y, LIU T, MEI Y, WANG Z. Application of general regression neural network to model a novel integrated fluidized bed gasifier[J]. Int J Hydrogen Energy, 2018, 43(11):5512-5521. doi: 10.1016/j.ijhydene.2018.01.130 [3] MEI Y, WANG Z, FANG Y, HUANG J, LI W, GUO S, LI G. CO2 catalytic gasification with NaAlO2 addition for its low-volatility and tolerant to deactivate[J]. Fuel, 2019, 242:160-166. doi: 10.1016/j.fuel.2019.01.014 [4] MIN Z, YIMSIRI P, ASADULLAH M, ZHANG S, LI C. Catalytic reforming of tar during gasification. Part II. Char as a catalyst or as a catalyst support for tar reforming[J]. Fuel, 2011, 90(7):2545-2552. doi: 10.1016/j.fuel.2011.03.027 [5] LI C. Some recent advances in the understanding of the pyrolysis and gasification behaviour of victorian brown coal[J]. Fuel, 2007, 86(12):1664-1683. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=9dd8e1cc925b5df85cc11ee7b35bf753 [6] SONOYAMA N, OKUNO T, HOSOKAI S, LI C, HAYASHI J. Interparticle desorption and re-adsorption of alkali and alkaline earth metallic species within a bed of pyrolyzing char from pulverized woody biomass[J]. Energy Fuels, 2006, 20:1294-1297. doi: 10.1021/ef050316y [7] ELDEEB A B, BRICHKIN V N, KURTENKOV R V, BORMOTOV I S. Extraction of alumina from kaolin by a combination of pyro- and hydro-metallurgical processes[J]. App Clay Sci, 2019, 172:146-154. doi: 10.1016/j.clay.2019.03.008 [8] SHEMI A, NDLOVU S, SIBANDA V, VAN L D. Extraction of alumina from coal fly ash using an acid leach-sinter-acid leach technique[J]. Hydrometallurgy, 2015, 157:348-355. doi: 10.1016/j.hydromet.2015.08.023 [9] SILVA I F, LOBO L S. Study of CO2 gasification of activated carbon catalysed by molybdenum oxide and potassium carbonate[J]. Fuel, 1986, 65:1400-1403. doi: 10.1016/0016-2361(86)90113-4 [10] DING L, ZHOU Z, GUO Q, HUO W, YU G. Catalytic effects of Na2CO3 additive on coal pyrolysis and gasification[J]. Fuel, 2015, 142:134-144. doi: 10.1016/j.fuel.2014.11.010 [11] TANG J, WANG J. Catalytic steam gasification of coal char with alkali carbonates:A study on their synergic effects with calcium hydroxide[J]. Fuel Process Technol, 2016, 142:34-41. doi: 10.1016/j.fuproc.2015.09.020 [12] ARNOLD R A, HABIBI R, KOPYSCINSKI J, HILL J M. Interaction of potassium and calcium in the catalytic gasification of biosolids and switchgrass[J]. Energy Fuels, 2017, 31(6):6240-6247. doi: 10.1021/acs.energyfuels.7b00972 [13] LOBO L S, FIGUEIREDO J L, BERNARDO C A. Carbon formation and gasification on metals. Bulk diffusion mechanism:A reassessment[J]. Catal Today, 2011, 178(1):110-116. https://www.sciencedirect.com/science/article/abs/pii/S0920586111005748 [14] 郭涛, 曹林涛, 黄中, 江建忠, 徐正泉.准东高钠煤燃烧利用技术研究[J].煤炭技术, 2015, 34(1):331-333. http://d.old.wanfangdata.com.cn/Periodical/mtjs201501116GUO Tao, CAO Lin-tao, HUANG Zhong, JIANG Jian-zhong, XU Zheng-quan. Research on using technology in Zhundong high sodium coal combustion[J]. Coal Technol, 2015, 34(1):331-333. http://d.old.wanfangdata.com.cn/Periodical/mtjs201501116 [15] 郭帅, 霍晓东, 宋双双, 蒋云峰, 赵建涛, 房倚天.高钠煤中钠的赋存形态研究[J].燃料化学学报, 2017, 45(10):1171-1177. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract19099.shtmlGUO Shuai, HUO Xiao-dong, SONG Shuang-shuang, JIANG Yun-feng, ZHAO Jian-tao, FANG Yi-tian. Occurrence modes of sodium species in sodium-rich coals[J]. J Fuel Chem Technol, 2017, 45(10):1171-1177. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract19099.shtml [16] BAI Y, LÜ P, LI F, SONG X, SU W, YU G. Investigation into Ca/Na compounds catalyzed coal pyrolysis and char gasification with steam[J]. Energy Convers Manage, 2019, 184:172-179. doi: 10.1016/j.enconman.2019.01.063 [17] 谭心, 李璇, 于长永.碱金属在石墨烯表面吸附-迁移行为的第一性原理研究[J].原子与分子物理学报, 2017, 34(3):555-562. doi: 10.3969/j.issn.1000-0364.2017.03.029TAN Xin, LI Xuan, YU Chang-yong. Adsorption and diffusion behavior of alkali metal adatoms on graphene:A first-principle study[J]. J Atom Mol Phys, 2017, 34(3):555-562. doi: 10.3969/j.issn.1000-0364.2017.03.029 [18] KOPYSCINSKI J, RAHMAN M, GUPTA R, MIMS C A, HILL J M. K2CO3 catalyzed CO2 gasification of ash-free coal. Interactions of the catalyst with carbon in N2 and CO2 atmosphere[J]. Fuel, 2014, 117:1181-1189. doi: 10.1016/j.fuel.2013.07.030 [19] 代松涛, 许慎启, 于广锁.煤气化反应动力学实验研究方法进展[J].煤炭转化, 2008, 31(3):86-91. doi: 10.3969/j.issn.1004-4248.2008.03.020DAI Song-tao, XU Shen-qi, YU Guang-suo. Study of the experimental methods of coal gasification kinetics[J]. Coal Convers, 2008, 31(3):86-91. doi: 10.3969/j.issn.1004-4248.2008.03.020 [20] 闫景春, 沈来宏, 蒋守席, 葛晖骏.高钠煤化学链燃烧特性及煤焦气化反应动力学研究[J].化工学报, 2019, 70(5):1913-1922. http://d.old.wanfangdata.com.cn/Periodical/hgxb201905028YAN Jing-chun, SHEN Lai-hong, JIANG Shou-xi, GE Hui-jun. Chemical looping combustion of high-sodium coal and gasification kinetics of coal cha[J]. J Chem Ind Eng, 2019, 70(5):1913-1922. http://d.old.wanfangdata.com.cn/Periodical/hgxb201905028 [21] QUYN D M, WU H, LI C. Volatilisation and catalytic effects of alkali and alkaline earth metallic species during the pyrolysis and gasification of Victorian brown coal. Part I. Volatilisation of Na and Cl from a set of NaCl-loaded samples[J]. Fuel, 2002, 81:143-149. doi: 10.1016/S0016-2361(01)00127-2 [22] LI X, HAYASHI J, LI C. FT-Raman spectroscopic study of the evolution of char structure during the pyrolysis of a Victorian brown coal[J]. Fuel, 2006, 85(12):1700-1707. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=5f1d929e70499f5b83966ba0dfc5e28c [23] LOBO L S, CARABINERO S. Catalytic carbon gasification:Understanding catalyst-carbon contact and rate jump behavior with air[J]. Fuel Process Technol, 2018, 179:313-318. doi: 10.1016/j.fuproc.2018.07.018 [24] LOBO L S, CARABINERO S. Kinetics and mechanism of catalytic carbon gasification[J]. Fuel, 2016, 183:457-469. doi: 10.1016/j.fuel.2016.06.115 [25] LOBO L S. Intrinsic kinetics in carbon gasification:Understanding linearity, "nanoworms" and alloy catalysts[J]. Appl Catal B:Environ, 2014, 148:136-143. [26] MSRSH H, YAN D S. Formation of active carbons from cokes using potassium hydroxide[J]. Carbon, 1984, 22(6):603-611. doi: 10.1016/0008-6223(84)90096-4 [27] HAYASHI J, KAZEHAYA A, MUROYAMA K, WATKINSON A. Preparation of activated carbon from lignin by chemical activation[J]. Carbon, 2000, 38:1873-1878. doi: 10.1016/S0008-6223(00)00027-0 [28] GAO Y, YUE Q, XU S, GAO B. Activated carbons with well-developed mesoporosity prepared by activation with different alkali salts[J]. Mater Lett, 2015, 146:34-36. doi: 10.1016/j.matlet.2015.01.161 [29] GUO S, JIANG Y, LIU T, ZHAO J, HUANG J, FANG Y. Investigations on interactions between sodium species and coal char by thermogravimetric analysis[J]. Fuel, 2018, 214:561-568 doi: 10.1016/j.fuel.2017.11.069 [30] MOLINA A, MONTOYA A, MONDRAGO'N F. CO2 strong chemisorption as an astimate of coal char gasfication reactivity[J]. Fuel, 1999, 78:971-977. doi: 10.1016/S0016-2361(98)00220-8 [31] 张丽园, 胡小英, 姜菲, 盛蒂, 王传虎, 吕长鹏.以生物质为碳源制备氧化石墨烯的研究[J].蚌埠学院学报, 2017, 6(6):59-62. http://d.old.wanfangdata.com.cn/Periodical/bbxyxb201706015ZAHNG Li-yuan, HU Xiao-ying, JIANG Fei, SHENG Di, WANG Chuang-hu, LV Chang-peng. Research on preparation of graphene oxide from biomass[J]. J Bengbu Univ, 2017, 6(6):59-62. http://d.old.wanfangdata.com.cn/Periodical/bbxyxb201706015 [32] 葛涛, 马祥梅.炼焦煤中碳、氧、氮、硫赋存特征的XPS研究[J].煤炭技术, 2018, 37(3):293-295. http://d.old.wanfangdata.com.cn/Periodical/mtjs201803111GE Tao, MA Xiang-mei. XPS study on occurrence characteristics of carbon, oxygen, nitrogen and sulfur in coking coal[J]. Coal Technol, 2018, 37(3):293-295. http://d.old.wanfangdata.com.cn/Periodical/mtjs201803111 [33] 郝伟哲, 王壮志, 张学军, 田艳红.碳纤维表面元素在高温下的演变规律[J].化工进展, 2017, 36(21):332-338. http://d.old.wanfangdata.com.cn/Periodical/hgjz2017z1047HAO Wei-zhe, WANG Zhi-zhuang, ZHANG Xue-jun, TIAN Yan-hong. Evolution of element on the surface of carbon fiber during heat treatment[J]. Chem Ind Eng Prog, 2017, 36(21):332-338. http://d.old.wanfangdata.com.cn/Periodical/hgjz2017z1047 [34] 范延臻, 王贞.活性炭表面化学[J].煤炭转化, 2000, 23(4):26-30. doi: 10.3969/j.issn.1004-4248.2000.04.007FAN Yan-zhen, WANG Zhen. Surface chemistry of activated carbon[J]. Coal Convers, 2000, 23(4):26-30. doi: 10.3969/j.issn.1004-4248.2000.04.007 [35] SUHAS, CARROTT P J, RIBEIRO C. Lignin-from natural adsorbent to activated carbon:A review[J]. Bioresour Technol, 2007, 98(12):2301-2312. doi: 10.1016/j.biortech.2006.08.008 [36] 郭俊春.煤基活性炭生产技术工艺及发展[J].生物化工, 2018, 4(5):134-136. doi: 10.3969/j.issn.2096-0387.2018.05.039GUO Jun-chun. Technology and development of coal based activated carbon production technology[J]. Biomass Chem Ind, 2018, 4(5):134-136. doi: 10.3969/j.issn.2096-0387.2018.05.039 [37] CHEN S G, YANG R T. Unified mechanism of alkali and alkaline earth catalyzed gasification reactions of carbon by CO2 and H2O[J]. Energy Fuels, 1997, 11:421-427. doi: 10.1021/ef960099o [38] DING L, ZHANG Y, WANG Z, HUANG J, FANG Y. Interaction and its induced inhibiting or synergistic effects during co-gasification of coal char and biomass char[J]. Bioresour Technol, 2014, 173:11-20. doi: 10.1016/j.biortech.2014.09.007 [39] 梅艳钢, 王志青, 方慧斌, 冯荣涛, 房倚天.燃烧与催化气化灰中铝溶出行为的研究[J].燃料化学学报, 2017, 45(4):394-399. doi: 10.3969/j.issn.0253-2409.2017.04.002MEI Yan-gang, WANG Zhi-qing, FANG Hui-bin, FENG Rong-tao, FANG Yi-tian. Comparison of leaching behaviors of aluminum in ash from combustion and catalytic gasification[J]. J Fuel Chem Technol, 2017, 45(4):394-399. doi: 10.3969/j.issn.0253-2409.2017.04.002 [40] MEI Y, WANG Z, FANG H, WANG Y, HUANG J, FANG Y. Na-containing mineral transformation behaviors during Na2CO3-catalyzed CO2 gasification of high-alumina coal[J]. Energy Fuels, 2017, 31(2):1235-1242. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=593a2418946dc24eeeb5179af11f1366 [41] 陈兆辉, 刘雷, 金亚丹, 吴丽锋, 武恒, 湛月平, 李克忠, 毕继诚.高灰熔点煤的加压催化气化:K2CO3催化活性及钾回收特性[J].化工学报, 2017, 68(5):2155-2161. http://d.old.wanfangdata.com.cn/Periodical/hgxb201705051CHEN Zhao-hui, LIU Lei, JIN Ya-dan, WU Li-feng, WU Heng, ZHAN Yue-ping, LI Ke-zhong, BI Ji-cheng. Pressurized catalytic gasification of high ash fusion temperature coal:Catalytic activity of K2CO3 and potassium recovery[J]. J Chem Ind Eng, 2017, 68(5):2155-2161. http://d.old.wanfangdata.com.cn/Periodical/hgxb201705051 [42] KIM Y, PARK J, JUNG D, MIYAWAKI J, YOON S. MOCHIDA I. Low-temperature catalytic conversion of lignite:2. Recovery and reuse of potassium carbonate supported on perovskite oxide in steam gasification[J]. J Ind Eng Chem, 2014, 20(1):194-201. http://cn.bing.com/academic/profile?id=5a8b36afe553c9214c3b83871f5cb835&encoded=0&v=paper_preview&mkt=zh-cn [43] YUAN X, FAN S, CHOI S, KIM H T, LEE K B. Potassium catalyst recovery process and performance evaluation of the recovered catalyst in the K2CO3-catalyzed steam gasification system[J]. Appl Energy, 2017, 195:850-860. doi: 10.1016/j.apenergy.2017.03.088 [44] GUO Y, LI Y, CHENG F, WANG M, WANG X. Role of additives in improved thermal activation of coal fly ash for alumina extraction[J]. Fuel Process Technol, 2013, 110:114-121. doi: 10.1016/j.fuproc.2012.12.003 [45] GUO Y, YAN K, CUI L, CHENG F. Improved extraction of alumina from coal gangue by surface mechanically grinding modification[J]. Powder Technol, 2016, 302:33-41. doi: 10.1016/j.powtec.2016.08.034 [46] POPA T, FAN M, ARGYLE M D, SLIMANE R, BELL D, TOWLER B. Catalytic gasification of a powder river basin coal[J]. Fuel, 2013, 103:161-170. doi: 10.1016/j.fuel.2012.08.049 [47] WANG Y, WANG Z, HUANG J, FANG Y. Catalytic gasification activity of Na2CO3 and comparison with K2CO3 for a high-aluminum coal char[J]. Energy Fuels, 2015, 29(11):6988-6998. doi: 10.1021/acs.energyfuels.5b01537 -





 下载:
下载: