Conversion of 4-ethylphenol to light aromatics on the Cr2O3/Al2O3 modified by phosphoric acid
-
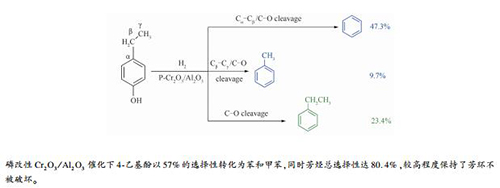
摘要: 以烷基酚转化为轻质芳烃(苯和甲苯)为目标,制备了Cr2O3/Al2O3催化剂,并以4-乙基酚为模型化合物研究了其加氢反应性能。体积空速、氢油比、反应压力和温度升高时,脱烷基率、芳烃总选择性、轻质芳烃选择性呈先增大后减小的趋势,反应温度对转化率影响较大。以不同浓度磷酸对Cr2O3/Al2O3进行改性,随着磷酸用量的增大,催化剂酸量总体增大,主要是弱酸和中强酸,酸强度先增加后降低,磷酸用量较高时,弱酸增加幅度较大。与未改性相比,质量分数8%磷酸改性Cr2O3/Al2O3上4-乙基酚转化率99.5%,脱烷基率提升9.4%,达74.4%,轻质芳烃选择性提高4.0%,达到57.0%,以较高选择性实现了转化制轻质芳烃,同时,芳烃总选择性高达80.4%,较高程度保持了芳环不被破坏。提出了Cr2O3/Al2O3上4-乙基酚加氢反应的路径并对反应机理进行了研究。Abstract: With the goal of conversion of alkylphenols to light aromatics (benzene and toluene), Cr2O3/Al2O3 catalysts were prepared and their hydrogenation performance was investigated using 4-ethylphenol as a model compound. With the increase of LHSV, H2/oil, reaction pressure and temperature, the dealkylation rate, the total selectivity of aromatics, and the selectivity of light aromatics first rose and then dropped. The conversion of 4-ethylphenol was obviously influenced by the reaction temperature. Cr2O3/Al2O3 was modified with different concentrations of phosphoric acid. As the increase of the amount of phosphoric acid, the general amount of weak and medium acids on the catalyst increased, and the strength of acid was first enhanced and then weakened. The amount of weak acid increased significantly under a high value of the amount of phosphoric acid. Compared with the unmodified catalyst, the conversion of 4-ethylphenol on the catalysts modified by 8% phosphoric acid is higher than 99.5%, while the dealkylation rate of 4-ethylphenol increased by 9.4%, reaching to 74.4%, and the selectivity to light aromatics (benzene and toluene) increased by 4.0%, reaching to 57.0%. Conversion of 4-ethylphenol to light aromatics was achieved in high selectivity. Furthermore, the total selectivity of aromatics was as high as 80.4%, which meant that most of the aromatic rings was not broken. The path of hydrogenation reaction of 4-ethylphenol on Cr2O3/Al2O3 was proposed and the reaction mechanism was discussed.
-
Key words:
- coal tar /
- 4-ethylphenol /
- phosphoric acid /
- alumina /
- chromium oxide
-
图 7 不同含量磷酸改性对Cr2O3/Al2O3催化4-乙基酚加氢反应性能的影响
Figure 7 Effect of modification with various content of phosphoric acid on the performance of the Cr2O3/Al2O3 catalysts for the hydrogenation of 4-ethylphenol
reaction conditions: LHSV=6 h-1, H2/oil=500 :1, p=3.5 MPa, t=450 ℃ P-0: 0 H3PO4; P-1: 4%H3PO4; P-2: 6%H3PO4; P-3: 8%H3PO4; P-4: 10%H3PO4; P-5: 12%H3PO4
图 8 不同含量磷酸改性Cr2O3/Al2O3催化剂反应前后的XRD谱图
Figure 8 XRD patterns of the Cr2O3/Al2O3 catalysts modified with various content of phosphoric acid before and after reaction
reaction conditions: LHSV=6 h-1, H2/oil=500 :1, p=3.5 MPa, t=450 ℃; AF: after P-0: 0 H3PO4; P-1: 4%H3PO4; P-2: 6%H3PO4; P-3: 8%H3PO4; P-4: 10%H3PO4; P-5: 12%H3PO4
表 1 不同含量磷酸改性Cr2O3/Al2O3催化剂的孔结构参数表
Table 1 Pore structure parameters of the Cr2O3/Al2O3 catalysts modified by various content of phosphoric acid
Catalyst Specific surface area
A/(m2·g-1)Pore volume
v/(cm3·g-1)Average pore size
d/nmP-1 140.6 0.44 12.5 P-2 148.8 0.47 12.5 P-3 146.7 0.47 12.7 P-4 134.5 0.41 12.1 P-5 131.9 0.40 10.4 reaction conditions:LHSV=6 h-1,H2/oil=500 :1,
p=3.5 MPa,t=450 ℃
P-1: 4%H3PO4;P-2: 6%H3PO4;P-3: 8%H3PO4;P-4: 10%H3PO4;P-5: 12%H3PO4表 2 不同含量磷酸改性Cr2O3/Al2O3催化剂表面元素相对百分含量
Table 2 Relative percentage content of surface elements of the Cr2O3/Al2O3 catalysts modified with different phosphoric acid content
Catalyst Content w/% P O Cr P-0 0.00 96.06 3.94 P-1 1.61 94.54 3.85 P-2 2.09 94.21 3.70 P-3 2.32 93.78 3.90 P-4 2.65 93.34 4.01 P-5 3.59 92.55 3.86 AFP-3 2.20 93.78 4.02 reaction conditions:LHSV=6 h-1,H2/oil=500 :1,p=3.5 MPa,t=450 ℃
P-0: 0 H3PO4;P-1: 4%H3PO4;P-2: 6%H3PO4;P-3: 8%H3PO4;P-4: 10%H3PO4;P-5: 12%H3PO4 -
[1] ZHAO N, LIU D, DU H, WEN F, SHI N. Investigation on component separation and structure characterization of medium-low temperature coal tar[J]. Appl Sci, 2019, 9(20):4335. doi: 10.3390/app9204335 [2] 张生娟, 高亚男, 陈刚, 姬鹏军, 石欣, 赵静, 赵丽信, 王艳红.煤焦油中酚类化合物的分离及其组成结构鉴定研究进展[J].化工进展, 2018, 37(7):139-147. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hgjz201807015ZANG Sheng-juan, GAO Ya-nan, CHEN Gang, JI Peng-jun, SHI Xin, ZHAO Jing, ZHAO Li-xin, WANG Yan-hong. Research progress on isolation of phenolic compounds from coal tar and its composition and structure identification[J]. Chem Ind Eng Prog, 2018, 37(7):139-147. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hgjz201807015 [3] YI L, FENG J, LI W, LUO Z. High-performance separation of phenolic compounds from coal-based liquid oil by deep eutectic solvents[J]. ACS Sustainable Chem Eng, 2019, 7(8):7777-7783. doi: 10.1021/acssuschemeng.8b06734 [4] SUN M, ZHANG D, YAO Q, LIU Y, SU X, JIA Charles Q, HAO Q, MA X. Separation and composition analysis of GC/MS analyzable and unanalyzable parts from coal tar[J]. Energy Fuels, 2018, 32(7):7404-7411. doi: 10.1021/acs.energyfuels.8b01054 [5] 李军芳, 毛学锋, 胡发亭.中低温煤焦油酚油馏分中酚类化合物的组成[J].煤炭转化, 2019, 42(2):32-38. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=mtzh201902006LI Jun-fang, MAO Xue-feng, HU Fa-ting. Composition of phenolic compounds in phenol oil distillate of medium and low temperature coal tar[J]. Coal Convers, 2019, 42(2):32-38. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=mtzh201902006 [6] 史军歌, 吴梅.气相色谱-氧选择性火焰离子检测器在煤焦油酚类化合物分析研究中的应用[J].石油炼制与化工, 2019, 50(7):97-102. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=sylzyhg201907019SHI Jun-ge, WU Mei. Application of gas chromatography-oxygen selective flame ion detector in analytical research of phenolic compounds in coal tar[J]. Pet Process Petrochem, 2019, 50(7):97-102. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=sylzyhg201907019 [7] 王汝成, 孙鸣, 刘巧霞, 马燕星, 冯光, 徐龙, 马晓迅.陕北中低温煤焦油中酚类化合物的提取与GC/MS分析[J].煤炭学报, 2011, 36(4):664-669. http://www.cqvip.com/QK/96550X/201104/37560728.htmlWANG Ru-cheng, SUN Ming, LIU Qiao-xia, MA Yan-xing, FENG Guang, XU Long, MA Xiao-xun. Extraction and GC/MS analysis of phenolic compounds in middle and low temperature coal tars in Northern Shaanxi[J]. J China Coal Soc, 2011, 36(4):664-669. http://www.cqvip.com/QK/96550X/201104/37560728.html [8] SHI L, ZHANG Z, QIU Z, GUO F, ZHANG W, ZHAO L. Effect of phosphorus modification on the catalytic properties of Mo-Ni/Al2O3 in the hydrodenitrogenation of coal tar[J]. J Fuel Chem Technol, 2015, 43(1):74-80. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rlhxxb201501012 [9] 胡乃方, 崔海涛, 邱泽刚, 赵亮富.不同P改性方式对Mo-Co/γ-Al2O3煤焦油加氢脱硫性能的影响[J].石油炼制与化工, 2016, 47(9):67-74. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=sylzyhg201609013HU Nai-fang, CUI Hai-tao, QIU Ze-gang, ZHAO Liang-fu. Effect of different P modification methods on the performance of Mo-Co/γ-Al2O3 coal tar hydrodesulfurization[J]. Pet Process Petrochem, 2016, 47(9):67-74. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=sylzyhg201609013 [10] FENG J, YANG Z, HSE C, SU Q, WANG K, JING J, XU J. In situ catalytic hydrogenation of model compounds and biomass-derived phenolic compounds for bio-oil upgrading[J]. Renewable Energy, 2017, 105:140-148. doi: 10.1016/j.renene.2016.12.054 [11] DE SOUZA P M, RABELO-NETO R C, BORGES L E P. Hydrodeoxygenation of phenol over Pd catalysts. Effect of support on reaction mechanism and catalyst deactivation[J]. ACS Catal, 2017, 7(3):2058-2073. doi: 10.1021/acscatal.6b02022 [12] LUO Z, ZHENG Z, WANG Y, SUN G, JIANG H, ZHAO C. Hydrothermally stable Ru/HZSM-5-catalyzed selective hydrogenolysis of lignin-derived substituted phenols to bio-arenes in water[J]. Green Chem, 2016, 18(21):5845-5858. doi: 10.1039/C6GC01971D [13] 鲁金芝, 魏雪梅, 马占伟, 胡斌.催化剂形态与酚类化合物加氢反应活性构效关系的研究进展[J].化工进展, 2020, 39(3):1000-1011. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hgjz202003023LU Jin-zhi, WEI Xue-mei, MA Zhan-wei, HU Bin. Structure-activity relationship of catalyst morphology and phenolic compound hydrogenation activity[J]. Chem Ind Eng Pro, 2020, 39(3):1000-1011. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hgjz202003023 [14] SUN Z, FRIDRICH B, DE SANTI A, ELANGOVAN S, BARTA K. Bright side of lignin depolymerization:Toward new platform chemicals[J]. Chem Rev, 2018, 118(2):614-678. doi: 10.1021/acs.chemrev.7b00588 [15] 纪娜, 宋静静, 刁新勇, 宋春风, 刘庆岭, 郑明远.硫化物催化木质素及其模型化合物转化制备高附加值化学品[J].化学进展, 201729(5):113-128. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hxjz201705011JI Na, SONG Jing-jing, DIAO Xin-yong, SONG Chun-feng, LIU Qing-ling, ZHENG Ming-yuan. Sulfide-catalyzed conversion of lignin and its model compounds to produce high value-added chemicals[J]. Prog Chem, 201729(5):113-128. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hxjz201705011 [16] SAIDI M, SAMIMI F, KARIMIPOURFARD D, NIMMANWUDIPONG T, GATES B C, RAHIMPOUR M R. Upgrading of lignin-derived bio-oils by catalytic hydrodeoxygenation[J]. Energy Environ Sci, 2014, 7(1):103-129. doi: 10.1039/C3EE43081B [17] 邱泽刚, 尹婵娟, 李志勤, 冯跃阔.酚类加氢脱氧催化剂研究进展[J].化工进展, 2019, 38(8):3658-3669. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hgjz201908020QIU Ze-gang, YIN Chan-juan, Li Zhi-qin, FENG Yue-kuo. Research progress on phenol hydrodeoxygenation catalysts[J].Chem Ind Eng Prog, 2019, 38(8):3658-3669. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hgjz201908020 [18] KATADA N, KAWAGUCHI Y, TAKEDA K, MATSUOKA T. Dealkylation of alkyl polycyclic aromatic hydrocarbon over silica monolayer solid acid catalyst[J]. Appl Catal A:Gen, 2017, 530:93-101. doi: 10.1016/j.apcata.2016.11.018 [19] AL-KHATTAF S S, ALI S A, AITANI A M. Fixed-bed alkyl-aromatic conversion process: US, 10173204[P]. 2019-1-8. [20] SHIN J, OH Y, CHOI Y, LEE J, LEE J K. Design of selective hydrocracking catalysts for BTX production from diesel-boiling-range polycyclic aromatic hydrocarbons[J]. Appl Catal A:Gen, 2017, 547:12-21. doi: 10.1016/j.apcata.2017.08.019 [21] 刘晨光, 刘欢, 殷长龙.高金属含量Ni-W催化剂的制备及竞争性催化反应性能[J].石油炼制与化工, 2014, 45(11):23-28. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=sylzyhg201411005LIU Chen-guang, LIU Huan, YIN Chang-long. Preparation of Ni-W catalyst with high metal content and competitive catalytic performance[J]. Pet Process Petrochem, 2014, 45(11):23-28. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=sylzyhg201411005 [22] 申群兵.负载金属氧化物和贵金属的分子筛催化剂上重芳烃加氢脱烷基制备BTX研究[D].上海: 华东理工大学, 2010.SHEN Qun-bing. Hydrogenation and dealkylation of heavy aromatics to BTX on molecular sieve catalysts supporting metal oxides and precious metals[D]. Shanghai: East China University of Science and Technology, 2010. [23] 王士文, 廖巧丽, 秦永宁.新型C9-C10芳烃脱烷基催化剂的研究[J].石油化工, 1995, (12):849-851. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QK199500484679WANG Shi-wen, LIAO Qiao-li, QIN Yong-ning. Research on new C9-C10 aromatic dealkylation catalyst[J]. Petrochem Ind, 1995, (12):849-851. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QK199500484679 [24] 潘志英.负载型HMCM-56催化剂对重芳烃加氢脱烷基催化性能的研究[D].上海: 华东理工大学, 2011.PAN Zhi-ying. Study on the Catalytic Performance of Supported HMCM-56 Catalyst for Hydrodealkylation of Heavy Aromatic Hydrocarbons[D]. Shanghai: East China University of Science and Technology, 2011. [25] VERBOEKEND D, LIAO Y, SCHUTYSER W, SELS B F. Alkylphenols to phenol and olefins by zeolite catalysis:A pathway to valorize raw and fossilized lignocellulose[J]. Green Chem, 2016, 18(1):297-306. http://www.researchgate.net/publication/283029145_Alkylphenols_to_phenol_and_olefins_by_zeolite_catalysis_A_pathway_to_valorize_raw_and_fossilized_lignocellulose [26] LESMANA D, WU H S. Cu/ZnO/Al2O3/Cr2O3/CeO2 catalyst for hydrogen production by oxidative methanol reforming via washcoat catalyst preparation in microchannel reactor[J]. Bull Chem React Eng Catal, 2017, 12(3):384-392. doi: 10.9767/bcrec.12.3.966.384-392 [27] ZHANG M, ZHAO R, LING Y, WANG R, ZHOU Q. Preparation of Cr2O3/Al2O3 bipolar oxides as hydrogen permeation barriers by selective oxide removal on SS and atomic layer deposition[J]. Int J Hydrogen Energy, 2019, 44(23):12277-12287. doi: 10.1016/j.ijhydene.2019.03.086 [28] BAII L, CARLTON JR D D, SCHUG K A. Complex mixture quantification without calibration using gas chromatography and a comprehensive carbon reactor in conjunction with flame ionization detection[J]. J Sep Sci, 2018, 41(21):4031-4037. doi: 10.1002/jssc.201800383 [29] MENG S, CHANG S, CHEN S. Synergistic effect of photocatalyst CdS and thermalcatalyst Cr2O3-Al2O3 for selective oxidation of aromatic alcohols into corresponding aldehydes[J]. ACS Appl Mater Interfaces, 2019. [30] XING R, FRIDMAN V, SEVERANCE M. Investigating the CrOx/Al2O3 dehydrogenation catalyst model: I. identification and stability evaluation of the Cr species on the fresh and equilibrated catalysts[J]. Appl Catal A: Gen. 2016, 523: 39-53. [31] DONG J, WANG J, WANG J, YANG M, LI W. Enhanced thermal stability of palladium oxidation catalysts using phosphate-modified alumina supports[J]. Catal Sci Technol, 2017, 7(21):5038-5048. doi: 10.1039/C7CY01534H [32] ZHAO Y, CHEN D K, LIU J P, HE D D, CAO X H, HAN C Y, LU J C, LUO Y M. Tuning the metal-support interaction on chromium-based catalysts for catalytically eliminate methyl mercaptan:Anchored active chromium species through surface hydroxyl groups[J]. Chem Eng J, 2020, 389:124384. doi: 10.1016/j.cej.2020.124384 [33] 胡乃方, 崔海涛, 邱泽刚, 赵亮富, 孟欣欣, 赵正权, 敖广宇.不同P负载量对Co-Mo/γ-Al2O3煤焦油加氢脱硫性能影响的研究[J].燃料化学学报, 2016, 44(6):745-753. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rlhxxb201606016HU Nai-fang, CUI Hai-tao, QIU Ze-gang, ZHAO Liang-fu, MENG Xin-xin, ZHAO Zheng-quan, AO Guang-yu. Effects of Different P. Loadings on the hydrodesulfurization performance of Co-Mo/γ-Al2O3 coal tar[J]. J Fuel Chem Technol, 2016, 44(6):745-753. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rlhxxb201606016 [34] HERRERA-GOMEZ A, CABRERA-GERMAN D, DUTOI A D. Intensity modulation of the Shirley background of the Cr3p spectra with photon energies around the Cr2p edge[J]. Surf Interface Anal, 2018, 50(2):246-252. [35] PARK J H, YEO S, KANG T J, I HEO, LEE K Y, CHANG T S. Enhanced stability of Co catalysts supported on phosphorus-modified Al2O3 for dry reforming of CH4[J]. Fuel, 2018, 212:77-87. doi: 10.1016/j.fuel.2017.09.090 [36] 戴厚良主编.芳烃技术[M].北京:中国石化出版社, 2014, 第1版, 259-260. [37] ČEJKA J, WICHTERLOVÁ B. Acid-catalyzed synthesis of mono-and dialkyl benzenes over zeolites:Active sites, zeolite topology, and reaction mechanisms[J]. Catal Rev, 2002, 44(3):375-421. doi: 10.1081/CR-120005741 [38] ROMERO Y, RICHARD F, BRUNET S. Hydrodeoxygenation of 2-ethylphenol as a model compound of bio-crude over sulfided Mo-based catalysts:Promoting effect and reaction mechanism[J]. Appl Catal B:Environ, 2010, 98(3/4):213-223. http://www.sciencedirect.com/science/article/pii/S0926337310002407 -





 下载:
下载: