-
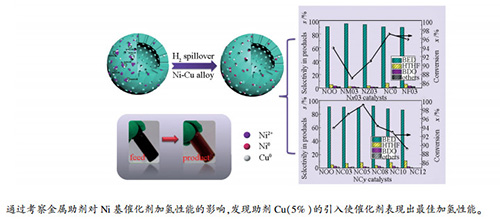
摘要: 采用沉积沉淀法将金属助剂引入Ni/Al2O3催化剂,考察不同金属助剂对于BYD加氢体系的影响,进一步优选助剂的最佳含量,并结合BET、XRD、H2-TPR、EDX-MAPPING、TEM、XPS、NH3-TPD等表征手段对催化剂物化性质进行研究。结果表明,金属助剂的添加主要影响了活性组分与载体间相互作用,成为影响催化活性的主要原因。Cu与Fe的引入使催化剂中Ni2+与载体之间相互作用明显减弱,提高了还原性能,BYD转化率提高至95%。通过考察优选金属助剂Cu含量对于催化剂物化性质的影响,发现使Ni2+与载体间相互作用力减弱的主要原因在于Cu表面氢溢流现象,然而,较多还原后的Ni颗粒由于与载体间的弱相互作用,易发生团聚,对加氢过程造成不利影响,通过Ni-Cu金属作用可有效地将金属固定于在载体表面,避免粒子迁移、团聚,Cu添加量5%时,催化剂凭借较多分散度良好的活性组分和适宜酸性,最终表现出最优加氢性能。
-
关键词:
- Ni/Al2O3催化剂 /
- Ni-Cu作用 /
- 改性 /
- 1, 4-丁炔二醇 /
- 1, 4-丁烯二醇
Abstract: The deposition-precipitation method was employed for the purpose of bringing in metallic promoters into Ni/Al2O3 catalysts. The effects of various metallic promoters on the catalytic performance in 1, 4-butynediol (BYD) hydrogenation were investigated. Besides, the contents of the suitable promoter were further studied, which were combined with BET, XRD, H2-TPR, EDX-MAPPING, TEM, XPS, and NH3-TPD techniques, aimed at exploring physico-chemical characteristics in catalysts. As the findings suggested, the addition of different promoters substantially impacted the interaction between Ni2+ and support, acting as the key factor impacting the catalytic performance. The introduction of Cu and Fe had the potential to prominently lower the strong interaction between Ni2+ and support for the improvement of the BYD conversion of 95%. Furthermore, different contents of Cu were further studied and discovered that it was the phenomenon of hydrogen spillover arisen on Cu surfaces, efficiently lowering the interaction between Ni2+ and support. Nevertheless, the aggregation in Ni particles cannot be evitable, but for the existence of Ni-Cu alloying in Cu-added catalysts. As the Cu content reached up to 5%, the catalyst manifested the excellent catalytic performance in hydrogenation owing to the abundant amount of Ni0 active sites in the form of the high dispersion and the fitting acidity.-
Key words:
- Ni/Al2O3 catalysts /
- Ni-Cu /
- modification /
- BYD /
- BED
-
表 1 不同Nx03催化剂的织构参数
Table 1 Textural properties of different Nx03 catalysts
Catalyst ABET/(m2·g-1) vp/(cm3·g-1) Dp/nm DNia/nm N00 154.28 0.54 14.00 9.0 NZ03 147.09 0.52 14.16 8.3 NM03 148.52 0.53 14.22 7.9 NF03 147.37 0.52 14.25 12.1 NC03 146.68 0.52 14.09 11.5 a: the mean sizes of nickel crystallites were determined from the broadening of the Ni (111), according to the Scherrer-Warren equation 表 2 不同NCy催化剂的织构参数
Table 2 Textural properties of different NCy catalysts
Catalyst ABET/(m2·g-1) vp/(cm3·g-1) Dp/nm N00 154.28 0.54 14.00 NC03 146.68 0.52 14.09 NC05 140.24 0.50 14.17 NC08 134.60 0.48 14.23 NC10 128.97 0.47 14.56 NC12 123.81 0.46 14.89 表 3 还原后不同NCy催化剂的XPS分析
Table 3 Detailed XPS results of the NCy catalysts after reduction
Catalyst Ni peak area ratio /% Cu peak area ratio /% Ni0 Ni2+a Ni2+b satellite Cu0/Cu+ Cu2+ satellite NC00 9.5 39.8 25.2 25.5 - - - NC03 9.6 44.9 16.1 29.4 48.9 51.1 - NC05 12.9 48.0 10.7 28.4 53.9 46.1 - NC08 13.5 45.0 13.0 28.5 58.0 27.7 14.3 NC10 14.7 40 14.0 31.3 60.3 27.2 12.5 NC12 15.1 47.4 7.0 30.5 62.0 24.3 13.7 a: Ni2+ weakly interacting with support; b: Ni2+ strongly interacting with support -
[1] 李长有, 杨乐夫, 蔡钒, 黄波, 胡昱翔, 蔡俊修.烃类化合物炔键选择加氢Pd-Pb/CaCO3催化剂的研究[J].厦门大学学报(自然科学版), 2005, (4):515-519. doi: 10.3321/j.issn:0438-0479.2005.04.018LI Chang-you, YANG Le-fu, CAI Fan, HUANG Bo, HU Yu-xiang, CAI Jun-xiu. Study on Pd-Pb/CaCO3 catalyst for selective hydrogenation of acetylenic link in hydrocarbon[J]. J Xiamen Univ (Nat Sci), 2005, (4):515-519. doi: 10.3321/j.issn:0438-0479.2005.04.018 [2] NATIVIDAD R, CRUZ OLIVARES J, FISHWICK R P, WOOD J, WINTERBOTTOM J M. Scaling-out selective hydrogenation reactions:From single capillary reactor to monolith[J]. Fuel, 2007, 86(9):1304-1312. doi: 10.1016/j.fuel.2006.12.005 [3] TELKAR M M, RODE C V, RANE V H, JAGANATHAN R, CHAUDHARI R V. Selective hydrogenation of 2-butyne-1, 4-diol to 2-butene-1, 4-diol:Roles of ammonia, catalyst pretreatment and kinetic studies[J]. Appl Catal A:Gen, 2001, 216(1):13-22. http://cn.bing.com/academic/profile?id=60a21a105967aa7df93ec536c7536b48&encoded=0&v=paper_preview&mkt=zh-cn [4] 刘响, 廖启江, 张敏卿. 1, 4-丁炔二醇加氢过程研究进展[J].化工进展, 2017, 36(8):2787-2797. http://d.old.wanfangdata.com.cn/Periodical/hgjz201708007LIU Xiang, LIAO Qi-jiang, ZHANG Min-qing. Research progress of 1, 4-butynediol hydrogenation process[J]. Chem Ind Eng Prog, 2017, 36(8):2787-2797. http://d.old.wanfangdata.com.cn/Periodical/hgjz201708007 [5] CHEN X, ZHANG M M, YANG K X, WILLIAMS C T, LIANG C H. Raney Ni-Si catalysts for selective hydrogenation of highly concentrated 2-butyne-1, 4-diol to 2-butene-1, 4-diol[J]. Catal Lett, 2014, 144(7):1118-1126. doi: 10.1007/s10562-014-1259-8 [6] TANIELYAN S, SCHIMIDT S, MARIN N, ALVEZ G, AUGUSTINE R. Selective hydrogenation of 2-butyne-1, 4-diol to 1, 4-butanediol over particulate raney(R) nickel catalysts[J]. Top Catal, 2010, 53(15/18):1145-1149. https://www.researchgate.net/publication/225527374_Selective_Hydrogenation_of_2-Butyne-14-diol_to_14-Butanediol_Over_Particulate_RaneyA_R_Nickel_Catalysts [7] LI C, ZHANG M, DI X, YIN D, LI W, LIANG C. One-step synthesis of Pt@ZIF-8 catalyst for the selective hydrogenation of 1, 4-butynediol to 1, 4-butenediol[J]. Chin J Catal, 2016, 37(9):1555-1561. doi: 10.1016/S1872-2067(16)62497-X [8] ZHANG M M, YANG Y B, LI C, LIU Q, WILLIAMS C T, LIANG C H. PVP-Pd@ZIF-8 as highly efficient and stable catalysts for selective hydrogenation of 1, 4-butynediol[J]. Catal Sci Technol, 2014, 4(2):329-332. doi: 10.1039/C3CY00873H [9] SAVVA P G, GOUNDANI K, VAKROS J, BOURIKAS K, FOUNTZOULA C, VATTIS D, LYCOURGHIOTIS A, KORDULIS C. Benzene hydrogenation over Ni/Al2O3 catalysts prepared by conventional and sol-gel techniques[J]. Appl Catal B:Environ, 2008, 79(3):199-207. doi: 10.1016/j.apcatb.2007.10.023 [10] XIONG J, CHEN J, ZHANG J. Liquid-phase hydrogenation of o-chloronitrobenzene over supported nickel catalysts[J]. Catal Commun, 2007, 8(3):345-350. doi: 10.1016/j.catcom.2006.06.028 [11] MICHALSKA K, KOWALIK P, KONKOL M, PROCHNIAK W, STOLECKI K, SLOWIK G, BOROWIECKI T. The effect of copper on benzene hydrogenation to cyclohexane over Ni/Al2O3 catalyst[J]. Appl Catal A:Gen, 2016, 523(5):54-60. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=e7fe2d78d1f227f4006c538df50e3b19 [12] PEYROVI M, TOOSI M. Study of benzene hydrogenation catalyzed by nickel supported on alumina in a fixed bed reactor[J]. React Kinet Catal Lett, 2008, 94(1):115-119. doi: 10.1007/s11144-008-5277-7 [13] LOULOUDI A, MICHALOPOULOS J, GANGAS N H, PAPAYANNAKOS N. Hydrogenation of benzene on Ni/Al-pillared saponite catalysts[J]. Appl Catal A:Gen, 2003, 242(1):41-49. doi: 10.1016/S0926-860X(02)00503-3 [14] ZIELINSKI M. The catalytic and physico-chemical properties of Ni/MgF2-MgO catalysts[J]. Appl Catal A:Gen, 2012, 449:15-22. doi: 10.1016/j.apcata.2012.09.033 [15] BOUDJAHEM A G, BOUDERBALA W, BETTAHAR M. Benzene hydrogenation over Ni-Cu/SiO2 catalysts prepared by aqueous hydrazine reduction[J]. Fuel Process Technol, 2011, 92(3):500-506. doi: 10.1016/j.fuproc.2010.11.003 [16] DONG X F, CAI X L, SONG Y B, LIN W M. Effect of transition metals (Cu, Co and Fe) on the autothermal reforming of methane over Ni/Ce0.2Zr0.1Al0.7Oδ catalyst[J]. J Nat Gas Chem, 2007, 16(1):31-36. doi: 10.1016/S1003-9953(07)60022-X [17] 李海涛.丁炔二醇加氢制1, 4-丁二醇催化剂的失活机理与改性研究[D].太原: 山西大学, 2011. http://cdmd.cnki.com.cn/Article/CDMD-10108-1012279648.htmLI Hai-tao. Study of deactivation and promotion of catalyst employed in hydrogenation of butynediol to 1, 4-butanediol[D].Taiyuan: Shanxi University, 2011. http://cdmd.cnki.com.cn/Article/CDMD-10108-1012279648.htm [18] KANG L N, GUO J Y, ZHANG H X, LI H T, XU Y L, ZHAO Y X. Activity and stability of Ni/SiO2-Al2O3 catalyst in the aqueous phase hydrogenation system[J]. J Mol Catal, 2014, 2:119-125. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=fzch201402004 [19] 任勇, 袁涛, 刘德蓉, 熊伟, 吴小海, 衣飞, 孟晓静. Pd-Cu/Fe3O4@C催化1, 4-丁炔二醇选择性加氢的研究[J].化学研究与应用, 2017, 29(11):1686-1692. doi: 10.3969/j.issn.1004-1656.2017.11.012REN Yong, YUAN Tao, LIU De-rong, XIONG Wei, WU Xiao-hai, YI Fei, MENG Xiao-jing. Study on selective hydrogenation of 1, 4-butynediol by Pd-Cu/Fe3O4@C catalyst[J]. Chem Res Appl, 2017, 29(11):1686-1692. doi: 10.3969/j.issn.1004-1656.2017.11.012 [20] 李霞, 胡玉方, 刘德蓉, 冯建, 熊伟, 宋发益. Ru-Cu/Fe3O4-TiO2催化剂上甘油氢解制备1, 2-丙二醇的研究[J].化学研究与应用, 2016, 28(5):629-635. doi: 10.3969/j.issn.1004-1656.2016.05.011LI Xia, HU Yu-fang, LIU De-rong, FENG Jian, XIONG Wei, SONG Fa-yi. Hydrogenolysis of glycerol to 1, 2-propanediol over Ru-Cu/Fe3O4-TiO2 catalyst[J]. Chem Res Appl, 2016, 28(5):629-635. doi: 10.3969/j.issn.1004-1656.2016.05.011 [21] BANG Y J, SEO J G, SONG I K. Hydrogen production by steam reforming of liquefied natural gas (LNG) over Ni-Al2O3 catalysts prepared by a sequential precipitation method:Effect of precipitation agent[J]. Int J Hydrogen Energy, 2009, 34(19):8053-8060. doi: 10.1016/j.ijhydene.2009.08.020 [22] CAI B, ZHOU X C, MIAO Y C, LUO J Y, PAN H, HUANG Y B. Enhanced catalytic transfer hydrogenation of ethyl levulinate to γ-valerolactone over a robust Cu-Ni bimetallic catalyst[J]. ACS Sustainable Chem Eng, 2017, 5(2):1322-1331. doi: 10.1021/acssuschemeng.6b01677 [23] WANG A, YIN H, LU H, XUE J, REN M, JIANG T. Effect of organic modifiers on the structure of nickel nanoparticles and catalytic activity in the hydrogenation of p-nitrophenol to p-aminophenol[J]. Langmuir, 2009, 25(21):12736-12741. doi: 10.1021/la901815b [24] BAYAT N, REZAEI M, MESHKANI F. Methane decomposition over Ni-Fe/Al2O3 catalysts for production of COx-free hydrogen and carbon nanofiber[J]. Int J Hydrogen Energy, 2016, 41(3):1574-1584. doi: 10.1016/j.ijhydene.2015.10.053 [25] ZENG Y, WANG Z, LIN W, SONG W, CHRISTENSEN J M, JENSEN A. Hydrodeoxygenation of phenol over Pd catalysts by in-situ generated hydrogen from aqueous reforming of formic acid[J]. Catal Commun, 2016, 82(5):46-49. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=e67a483cd9c681f46a39eaaf1e2a61ca [26] CHEN J, QIAO Y, LI Y. Promoting effects of doping ZnO into coprecipitated NiAl2O3catalyst on methane decomposition to hydrogen and carbon nanofibers[J]. Appl Catal A:Gen, 2008, 337(2):148-154. doi: 10.1016/j.apcata.2007.12.007 [27] YADAV G D, KHARKARA M R. Liquid-phase hydrogenation of saturated and unsaturated nitriles:Activities and selectivities of bimetallic nickel-copper and Nickel-Iron catalysts supported on silica[J]. Appl Catal A:Gen, 1995, 126(1):115-123. doi: 10.1016/0926-860X(95)00039-9 [28] DONG F, ZHU Y, ZHENG H, ZHU Y, LI X, LI Y. Cr-free Cu-catalysts for the selective hydrogenation of biomass-derived furfural to 2-methylfuran:The synergistic effect of metal and acid sites[J]. J Mol Catal A:Chem, 2015, 398:140-148. doi: 10.1016/j.molcata.2014.12.001 [29] WANG Z, LIU Q, YU J, WU T, WANG G. Surface structure and catalytic behavior of silica-supported copper catalysts prepared by impregnation and Sol-Gel methods[J]. Appl Catal A:Gen, 2003, 239(1/2):87-94. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=361e9b97ad3ff83e3addcce23db04540 [30] ASHOK J, SUBRAHMANYAM M, AKULA V. Hydrotalcite structure derived Ni-Cu-Al catalysts for the production of H2 by CH4 decomposition[J]. Int J Hydrogen Energy, 2008, 33(11):2704-2713. doi: 10.1016/j.ijhydene.2008.03.028 [31] JUSZCZYK W, COLMENARES J C, ŚREBOWATA A, KARPINSKI Z. The effect of copper and gold on the catalytic behavior of nickel/alumina catalysts in hydrogen-assisted dechlorination of 1, 2-dichloroethane[J]. Catal Today, 2011, 169(1):186-191. doi: 10.1016/j.cattod.2010.08.019 [32] SHALI N B, SUGUNAN S. Influence of transition metals on the surface acidic properties of titania prepared by sol-gel route[J]. Mater Res Bull, 2007, 42(9):1777-1783. doi: 10.1016/j.materresbull.2006.11.016 [33] MORE S, TANIELYAN S L, AUGUSTINE R, SCHMIDT S. Continuous liquid phase hydrogenation of 1, 4-butynediol to high purity 1, 4-Butanediol over particulate catalyst in fixed bed reactor[J]. Org Process Res Dev, 2016, 21:327-335. https://www.researchgate.net/publication/299949885_Continuous_Liquid_Phase_Hydrogenation_of_14-Butynediol_to_High_Purity_14-Butanediol_Over_Particulate_Catalyst_In_Fixed_Bed_Reactor [34] KHOUZ M, GKANAS E I, DU S, WOOD J. Catalytic performance of Ni-Cu/Al2O3 for effective syngas production by methanol steam reforming[J]. Fuel, 2018, 232:672-683. doi: 10.1016/j.fuel.2018.06.025 [35] RIAZ N, KAIT C B, MAN Z, DUTTA B, KHAN M. Photodegradation of orange Ⅱ under visible light using Cu-Ni/TiO2:Influence of Cu:Ni mass composition, preparation, and calcination temperature[J]. Ind Eng Chem Res, 2013, 52(12):4491-4503. doi: 10.1021/ie303255v [36] YANG Z, LIU Y, LIU D, MENG X, LIU C. Hydroisomerization of n-octane over bimetallic Ni-Cu/SAPO-11 catalysts[J]. Catal Sci Technol, 2018, 8(3):817-828. doi: 10.1039/C7CY02106B [37] WU Q D L, DUCHSTEIN L, CHIARELLO G L, CHRISTENSEN J M, DAMSGAARD C, ELKJAR C, WAGNER J, TEMEL B, GRUNWALDT J D, JENSEN A. In situ observation of Cu-Ni alloy nanoparticle formation by X-Ray diffraction, X-Ray absorption spectroscopy, and transmission electron microscopy:Influence of Cu/Ni ratio[J]. ChemCatChem, 2013, 6(1):301-310. [38] HAO Z, ZHU Q, JIANG Z, HOU B, LI H. Characterization of aerogel Ni/Al2O3 catalysts and investigation on their stability for CH4-CO2 reforming in a fluidized bed[J]. Fuel Process Technol, 2009, 90(1):113-121. doi: 10.1016/j.fuproc.2008.08.004 [39] YANG Z, LIU Y, LIU D, MENG X, LIU C. Hydroisomerization of n-octane over bimetallic Ni-Cu/SAPO-11 catalysts[J]. Appl Catal A:Gen, 2017, 543:274-282. doi: 10.1016/j.apcata.2017.06.028 [40] MIRYALA B, REKHA V, PRASAD P S S, PRASAD R B N, NAKKA L. Selective hydrogenolysis of glycerol to 1, 2 propanediol over Cu-ZnO catalysts[J]. Catal Lett, 2008, 126(1/2):119-124. http://cn.bing.com/academic/profile?id=d375d9eeaee75c106ec0dce60fc6034e&encoded=0&v=paper_preview&mkt=zh-cn [41] SOUZA G D, BALZARETTI N M, MARCILIO N R, PEREZ-LOPEZ O W. Decomposition of ethanol over Ni-Al catalysts:Effect of copper addition[J]. Procedia Eng, 2012, 42:335-345. doi: 10.1016/j.proeng.2012.07.425 [42] KAMEOKA S, TANABE T, PANG T A. Spinel CuFe2O4:A precursor for copper catalyst with high thermal stability and activity[J]. Catal Lett, 2005, 100(1/2):89-93. https://www.researchgate.net/publication/244494479_Spinel_CuFe2O4_A_precursor_for_copper_catalyst_with_high_thermal_stability_and_activity [43] PRAKASH M G, MAHALAKSHMY R, KRISHNAMURTHY K R, VISWANATHAN B. Studies on Ni-M (M=Cu, Ag, Au) bimetallic catalysts for selective hydrogenation of cinnamaldehyde[J]. Catal Today, 2015, 263:105-111. [44] 王达, 张因, 李海涛, 赵丽丽, 张鸿喜, 赵永祥. Ni-Cu/Al2O3催化剂上顺酐液相选择加氢制丁二酸酐反应性能[J].催化学报, 2012, 33(7):1229-1235. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=cuihuaxb201207025WANG Da, ZHANG Yin, LI Hai-tao, ZHAO Li-li, ZHANG Hong-xi, ZHAO Yong-xiang. Selective hydrogenation of maleic anhydride to succinic anhydride in liquid phase over Ni-Cu/Al2O3 catalyst[J]. Chin J Catal, 2012, 33(7):1229-1235. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=cuihuaxb201207025 [45] IRIONDO A, CAMBRA J F, GUEMEZ M B, BARRIO V L, REQUIES J, SANCHEZ M C, NAVARRO R M. Effect of ZrO2 addition on Ni/Al2O3 catalyst to produce H2 from glycerol[J]. Int J Hydrogen Energy, 2012, 37(8):7084-7093. doi: 10.1016/j.ijhydene.2011.11.075 [46] SHI Y, CHEN W, DONGg L, LI H, FU Y. Enhancing copper infiltration into alumina using spark plasma sintering to achieve high performance Al2O3/Cu composites[J]. Ceram Int, 2018, 44(1):57-64. doi: 10.1016/j.ceramint.2017.09.062 [47] PRAKRUTHI H R, CHANDRASHEKARA B M, JAI PRAKASH B S, BHAT Y S. Hydrogenation efficiency of highly porous Cu-Al oxides derived from dealuminated LDH in the conversion of furfural to furfuryl alcohol[J]. J Ind Eng Chem, 2018, 62:96-105. doi: 10.1016/j.jiec.2017.12.048 [48] NAGHASH A R, ETSELL T H, XU S. XRD and XPS study of Cu-Ni interactions on reduced copper-nickel-aluminum oxide solid solution catalysts[J]. Chem Mater, 2006, 18(10):2480-2488. doi: 10.1021/cm051910o [49] XU S, WANG X. Highly active and coking resistant Ni/CeO2-ZrO2 catalyst for partial oxidation of methane[J]. Fuel, 2005, 84(5):563-567. doi: 10.1016/j.fuel.2004.10.008 -





 下载:
下载: