Effects of calcination atmosphere on the structure and performance of MoO3-SnO2 catalyst for the oxidation of dimethyl ether at low temperature
-
摘要: 采用共沉淀法制备了Mo/Sn物质的量比为1:3的MoO3-SnO2催化剂,考察了焙烧气氛(O2、air、N2和H2)对催化剂结构及二甲醚(DME)低温氧化制甲酸甲酯(MF)性能的影响。结果表明,在O2中焙烧的催化剂上DME转化率高达25.10%,MF选择性为72.21%,催化剂具有较高的反应活性。而在H2中焙烧催化剂上DME转化率仅为7.01%,MF选择性为75.82%。不同气氛焙烧催化剂上DME转化率由大到小的顺序:O2 > air> N2> H2。采用XRD、Raman、XPS及ESR等对催化剂进行深入表征。结果表明,共沉淀制备Mo1Sn3催化剂中钼物种以高分散MoOx形式存在。O2中焙烧催化剂表面Mo=O及存在于Mo-Sn界面处五配位钼氧化物中Mo5+含量均高于其他三种催化剂,即低聚态MoOx末端Mo=O可能是反应活性位点之一,五配位钼氧化物中Mo5+的存在有利于催化剂活性的提高,也有助于MF的生成。结合in suit DRIFTS证实了吸附于Mo5+上的CH3O,在催化剂表面Mo=O作用下氧化为HCHO后与另一分子CH3O耦合为MF。
-
关键词:
- MoO3-SnO2催化剂 /
- 焙烧气氛 /
- 二甲醚氧化 /
- 甲酸甲酯
Abstract: MoO3-SnO2 catalysts with a Mo/Sn molar ratio of 1:3 was prepared by the co-precipitation method and calcined in different atmospheres (O2, air, N2 and H2); the effect of calcination atmosphere on the catalytic performance of MoO3-SnO2 in the oxidation of dimethyl ether (DME) to methyl formate (MF) was investigated. The results show that the MoO3-SnO2 catalyst prepared by calcination in O2 exhibits the highest activity; the conversion of DME reaches 25.10%, with the selectivity of 72.21% to MF. Over the catalyst calcined in H2, the conversion of DME is only 7.01%, with the selectivity of 75.82% to MF. The activity of the MoO3-SnO2 catalysts calcined at different atmospheres follows the order of O2 > air > N2 > H2. The results of XRD, Raman, XPS and ESR characterization indicate the presence of MoOx domains on the surface of the MoO3-SnO2 catalyst with a Mo/Sn molar ratio of 1:3. The terminal Mo=O groups of oligomeric MoO3 may be the active sites for the methoxy intermediate and the penta-coordinated Mo5+ species in the Mo-Sn interface may be able to promote the oxidation of DME to MF. Consequently, methoxy species are absorbed on the Mo5+ species in the Mo-Sn interfaces, which are oxidized to HCHO on the terminal Mo=O groups; after that, the absorbed HCHO may then react with the neighboring absorbed methoxy species, forming MF.-
Key words:
- MoO3-SnO2 catalysts /
- calcination atmosphere /
- dimethyl ether oxidation /
- methyl formate
-
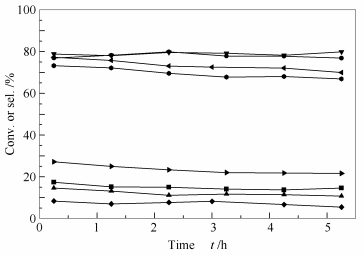
图 1 二甲醚在不同焙烧气氛MoO3-SnO2催化剂上的选择性氧化性能
Figure 1 Performance of the Mo1Sn3 catalyst calcined in different atmospheres for the oxidation of dimethyl ether (DME) to methyl formate (MF)
the reactions were conducted at atmospheric pressure and 150 ℃, with GHSV of 1 800 h-1 and DME/O2 ratio of 1:1 for MF selectivity: ▼: N2; ▶: air; ◀: H2; : O2; for DME conversion: ▶: O2; ■: air; ▲: N2; ◆: H2
表 1 反应温度对空气气氛中焙烧Mo1Sn3催化剂上DME的氧化反应性能
Table 1 Performance of the Mo1Sn3 catalyst calcined in air for the oxidation of dimethyl ether (DME) to methyl formate (MF) under different temperatures
Temperature
t/℃DME conversion
x/%Product selectivity s/% MF FA CH3OH CH4 CO CO2 140 12.40 80.35 0.09 12.06 0.00 7.50 0.00 150 17.42 77.00 0.02 7.68 0.00 15.30 0.00 160 97.72 0.12 0.00 0.06 0.00 99.82 0.00 reaction conditions: atmospheric pressure, GHSV=1 800 h-1, DME:O2= 1:1 表 2 反应空速对空气气氛中焙烧Mo1Sn3催化剂上DME的氧化反应性能
Table 2 Performance of the Mo1Sn3 catalyst calcined in air for the oxidation of dimethyl ether (DME) to methyl formate (MF) under different space velocities (GHSV)
GHSV /h-1 DME conversion
x/%Product selectivity s/% MF FA CH3OH CH4 CO CO2 900 25.50 75.95 0.01 5.10 0.00 18.94 0.00 1 800 17.42 77.00 0.02 7.68 0.00 15.30 0.00 3 600 14.69 68.23 6.76 13.31 0.00 11.70 0.00 reaction conditions: atmospheric pressure, temperature=150 ℃, DME:O2=1:1 表 3 反应温度对氧气气氛中焙烧Mo1Sn3催化剂上DME的氧化反应性能
Table 3 Performance of the Mo1Sn3 catalyst calcined in oxygen for the oxidation of dimethyl ether (DME) to methyl formate (MF) under different temperatures
Temperature
t/℃DME conversion
x/%Product selectivity s/% MF FA CH3OH CH4 CO CO2 120 16.58 76.57 0.16 23.27 0.00 0.00 0.00 130 16.78 81.92 0.03 10.81 0.00 7.24 0.00 140 14.47 81.02 0.05 9.11 0.00 9.82 0.00 150 21.23 74.88 0.03 9.64 0.00 15.45 0.00 160 25.92 70.83 0.01 7.07 0.00 22.09 0.00 reaction conditions: atmospheric pressure, GHSV=1 800 h-1, DME:O2=1:1 表 4 不同焙烧气氛MoO3-SnO2催化剂的XPS表征
Table 4 XPS results of the MoO3-SnO2 catalysts prepared with different calcination atmospheres
Catalyst O 1s E/eV Sn 3d E /eV Mo 3d E /eV Olat/(Osur+Olat) Osur Olat Sn 3d3/2 Sn 3d5/2 Mo 3d3/2 Mo 3d5/2 Mo1Sn3-O2 533.3 530.8 495.3 486.8 236.0 232.9 0.77 Mo1Sn3-air 533.1 530.3 494.9 486.3 235.6 232.5 0.73 Mo1Sn3-N2 532.7 530.1 494.5 486.1 235.3 232.2 0.78 Osur =surface active oxygen; surface hydroxyl oxygen and chemisorbed oxygen;Olat = lattice oxygen -
[1] WANG D S, HAN Y Z, TAN Y S, TSUBAKI N. Effect of H2O on Cu-based catalyst in one-step slurry phase dimethyl ether synthesis[J]. Fuel Process Technol, 2009, 90(3): 446-451. doi: 10.1016/j.fuproc.2008.11.007 [2] ZHANG Z Z, ZAHNG Q D, HAN Y Z, TSUBAKI N, TAN Y S. The effects of the Mo-Sn contact interface on the oxidation reaction of dimethyl ether to methyl formate at a low reaction temperature[J]. Catal Sci Technol, 2016, 6(15): 6109-6117. doi: 10.1039/C6CY00460A [3] ZHANG Z Z, ZAHNG Q D, HAN Y Z, TSUBAKI N, TAN Y S. Effect of MoO3 crystalline structure of MoO3-SnO2 catalysts on selective oxidation of glycol dimethyl ether to 1, 2-propandiol[J]. Catal Sci Technol, 2016, 6(6): 1842-1849. doi: 10.1039/C5CY00894H [4] 曹平, 杨先贵, 唐聪明, 王公应. MoO3催化碳酸二甲酯与乙酸苯酯合成碳酸二苯酯[J].催化学报, 2009, 30(9): 853-855. http://www.oalib.com/paper/4477369CAO Ping, YANG Xian-gui, TANG Cong-ming, WANG Gong-yun. Molybdenum trioxide catalyst for transesterification of dimethyl carbonate and phenyl acetate to diphenyl carbonate[J]. Chin J Catal, 2009, 30(9): 853-855. http://www.oalib.com/paper/4477369 [5] 刘金龙, 朱银华, 汪怀远. MoO3/TiO2催化剂的二苯并噻吩加氢脱硫性能[J].过程工程学报, 2009, 9(5): 882-886. http://www.cqvip.com/qk/94710a/200905/31794189.htmlLIU Jin-long, ZHU Yin-hua, WANG Huai-yuan. Hydrodesulfurization of dibenzothiophen with MoO3/TiO2 catalyst[J]. Chin J Process Eng, 2009, 9(5): 882-886. http://www.cqvip.com/qk/94710a/200905/31794189.html [6] LIU H C, CHEUNG P, IGLESIA E. Structure and support effects on the selective oxidation of dimethyl ether to formaldehyde catalyzed by MoOx domains[J]. J Catal, 2003, 217(1): 222-232. http://www.sciencedirect.com/science/article/pii/S0021951703000253 [7] LIU H C, IGLESIA E. Selective oxidation of dimethylether to formaldehyde on small molybdenum oxide domains[J]. J Catal, 2002, 208(1): 1-5. doi: 10.1006/jcat.2002.3574 [8] 黄秀敏, 徐奕德, 申文杰.负载型MoOx和VOx催化剂上二甲醚选择氧化制甲醛反应[J].催化学报, 2004, 25(4): 267-271. http://cdmd.cnki.com.cn/Article/CDMD-80038-2006122745.htmHUANG Xiu-min, XU Yi-de, SHEN Wen-jie. Selective oxidation of dimethylether to formaldehyde over supported MoOx and VOx catalysts[J]. Chin J Catal, 2004, 25(4): 267-271. http://cdmd.cnki.com.cn/Article/CDMD-80038-2006122745.htm [9] HUANG X M, LIU J L, CHEN J L, XU Y D, SHEN W J. Mechanistic study of selective oxidation of dimethyl ether to formaldehyde over Alumina-supported molybdenum oxide catalyst[J]. Catal Lett, 2006, 108(1/2): 79-86. http://www.springerlink.com/index/R16886210U312784.pdf [10] VALENTE N G. Structure and activity of Sn-Mo-O catalysts: Partial oxidation of methanol[J]. Appl Catal A: Gen, 2001, 205(1/2): 201-214. http://www.sciencedirect.com/science/article/pii/S0926860X00005652 [11] 刘广波, 张清德, 韩怡卓, 椿范立, 谭猗生. MoO3-SnO2催化剂上二甲醚低温氧化高选择性制备甲酸甲酯[J].燃料化学学报, 2013, 41(2): 223-227. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18126.shtmlLIU Guang-bo, ZAHNG Qing-de, HAN Yi-zhuo, CHUN Fan-li, TAN Yi-sheng. Low-temperature oxidation of dimethyl ether to methyl formate with high selectivity over MoO3-SnO2 catalysts[J]. J Fuel Chem Technol, 2013, 41(2): 223-227. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18126.shtml [12] LIU G B, ZHANG Q D, HAN Y Z, TAN Y S. Direct oxidation of dimethyl ether to ethanol over WO3/HZSM-5 catalysts[J]. Catal Commun, 2012, 26(35): 173-177. https://www.researchgate.net/publication/257630298_Direct_oxidation_of_dimethyl_ether_to_ethanol_over_WO3HZSM-5_catalysts [13] LIU G B, ZHANG Q D, HAN Y Z, TSUBAKI N, TAN Y S. Effects of the MoO3 structure of Mo-Sn catalysts on dimethyl ether oxidation to methyl formate under mild conditions[J]. Green Chem, 2015, 17(2): 1057-1064. doi: 10.1039/C4GC01591F [14] LIU G B, ZHANG Q D, HAN Y Z, TSUBAKI N, TAN Y S. Selective oxidation of dimethyl ether to methyl formate over trifunctional MoO3-SnO2 catalyst under mild conditions[J]. Green Chem, 2013, 15(6): 1501-1504. doi: 10.1039/c3gc40279g [15] ZHANG Z Z, ZAHNG Q D, HAN Y Z, TSUBAKI N, TAN Y S. Effect of tetrahedral molybdenum oxide species and MoOx domains on the selective oxidation of dimethyl ether under mild condition[J]. Catal Sci Technol, 2016, 6(9): 2975-2983. doi: 10.1039/C5CY01569C [16] COSIMO J I, MARCHI A J, APESTEGUIA C R. Preparation of ternary Cu/Co/Al catalysts by the amorphous citrate process[J]. J Catal, 1992, 134(2): 594-607. doi: 10.1016/0021-9517(92)90345-I [17] 王琪, 郝影娟, 陈爱平, 杨意泉.热处理对高硫化氢合成气一步法制甲硫醇K2MoO4-NiO/SiO2催化剂结构及性能的影响[J].催化学报, 2010, 31(2): 242-247.WANG Qi, HAO Ying-juan, CHEN Ai-ping, YANG Yi-quan. Effect of thermal treatment on structure and catalytic performance of K2MoO4-NiO/SiO2 catalyst for one-step synthesis of methanethiol from high H2S-containing syngas[J]. Chin J Catal, 2010, 31(2): 242-247. [18] NIWA M, YAMADA H, MURAKAMI Y. Activity for the oxidation of methanol of a molybdena monolayer supported on tin oxide[J]. J Catal, 1992, 134(1): 331-339. doi: 10.1016/0021-9517(92)90232-7 [19] STAMPF S, CHEN Y, DUMESIC J A, HILL C G. Interactions of molybdenum oxide with various oxide supports: Calcination of mechanical mixtures[J]. J Catal, 1987, 105(2): 445-454. doi: 10.1016/0021-9517(87)90072-8 [20] MENG Y L, WANG T, CHEN S, GONG J L. Selective oxidation of methanol to dimethoxymethane on V2O5-MoO3/γ-Al2O3 catalysts[J]. Appl Catal B: Environ, 2014, 160-161(1): 161-172. http://www.sciencedirect.com/science/article/pii/S0926337314002847 [21] VALENTE N G, ARR'UA L A, CAD'US L E. Structure and activity of Sn-Mo-O catalysts: Partial oxidation of methanol[J]. Appl Catal A: Gen, 2001, 205(1/2): 201-214. http://www.sciencedirect.com/science/article/pii/S0926860X00005652 [22] RUSLAN N N, TRIWAHYONO S, JALIL A A, TIMMIATI S N, ANNUAR N H R. Study of the interaction between hydrogen and the MoO3-ZrO2 catalyst[J]. Appl Catal A: Gen, 2012, 413(414): 176-182. http://www.sciencedirect.com/science/article/pii/S0926860X1100665X [23] SOJKA Z, CHE M. Catalytic chemistry of transition metal ions on oxide surfaces. A molecular approach using EPR techniques[J]. C R Acad Sci, Ser Ⅱc: Chim, 2000, 3(3): 163-174. http://www.sciencedirect.com/science/article/pii/S1387160900001389 [24] LOCHAR V. Study of methanol, formaldehyde and methyl formate adsorption on the surface of Mo/Sn oxide catalyst[J]. Appl Catal A: Gen, 2006, 309(1): 33-36. doi: 10.1016/j.apcata.2006.04.030 [25] WHITING G T, KONDRAT S A, HAMMOND C, DIMITRATOS N, HUTCHINGS G J. Methyl formate formation from methanol oxidation using supported Gold-Palladium nanoparticles[J]. ACS Catal, 2015, 5(2): 637-644. doi: 10.1021/cs501728r -






 下载:
下载: