Iron salts-catalyzed biomass hydropyrolysis for production of bio-oil and gaseous hydrocarbons
-
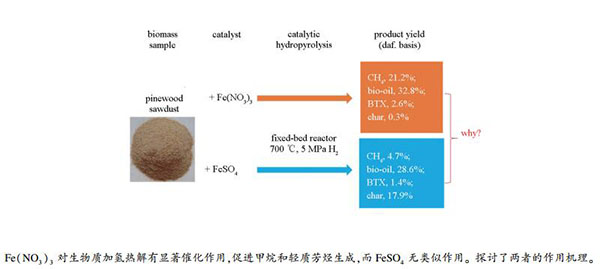
摘要: 利用加压固定床反应器进行了松木催化加氢热解实验(终温600-700 ℃、氢压5.0 MPa),考察了硝酸铁和硫酸亚铁两种铁盐对热解产物产率及分布的影响。研究发现,Fe(NO3)3能够显著促进生物炭加氢生成甲烷,碳转化率高达97.4%,CH4产率达21.2%,无水生物油产率为32.8%(产率基准均为干燥无灰生物质),生物油中含氧量降低,轻质芳烃产率增加,其中,苯、甲苯和二甲苯(BTX)产率为2.6%。而FeSO4迥异于Fe(NO3)3,具有抑制气态烃和生物油生成的作用。机理研究表明,Fe(NO3)3在加氢过程中主要形成α-Fe,并促使生物炭形成无定型和多孔结构,从而有利于其加氢生成甲烷,而FeSO4则部分转化为Fe2S3,由此可致使铁催化剂失活。Abstract: The catalytic hydropyrolysis of pine wood was conducted in a fixed bed reactor under a H2 pressure of 5 MPa at different temperatures (600-700 ℃) to investigate the effects of two iron salts, Fe(NO3)3 and FeSO4, on the upgrading of bio-oil and gaseous products. Fe(NO3)3 is found to promote the conversion of biomass to bio-oil and gaseous products, with a carbon conversion rate as high as 97.4%, a CH4 yield of 21.2%, and a bio-oil yield of 32.8% (daf. biomass basis). Moreover, the oxygen content in the bio-oil decreases, the yield of light aromatic hydrocarbon increases and the yield of BTX (benzene, toluene and xylene) reaches 2.6%. In contrast, FeSO4 has an inhibitory effect on the production of gaseous hydrocarbons and bio-oil. The XRD analysis shows that Fe(NO3)3 is transformed to α-Fe during hydropyrolysis, with the amorphous and porous structures of bio-char being formed. This is highly conducive to the catalytic hydrogenation and methanation of bio-char. But FeSO4 is converted to Fe2S3 during the hydropyrolysis, which might poison the catalytic activity.
-
Key words:
- biomass /
- hydropyrolysis /
- iron-based catalyst /
- bio-oil /
- methane
-
图 1 实验装置和流程示意图
Figure 1 Schematic diagram of the apparatus used for biomass catalytic hydropyrolysis
1: H2 gas cylinders; 2: pressure regulators; 3: dehumidifier; 4: mass flow controllers; 5: check valves; 6: ball valves; 7: reactor; 8: furnace; 9: samples; 10: thermocouple; 11: liquid product trap; 12: liquid nitrogen or ice- salt mixture; 13: pressure transducers; 14: mass flow meter; 15: gas chromatograph
图 6 无催化剂和含有两种铁基催化剂样品的热重-质谱分析(热重条件对应图 5)
Figure 6 TG-MS spectra of the pinewood devolatilization with no catalyst and with Fe(NO3)3 or FeSO4 catalyst
a: pinewood with NC; b: pinewood with Fe(NO3)3 catalyst; c: pinewood with FeSO4 catalyst
表 1 松木的工业分析和元素分析
Table 1 Proximate and ultimate analyses of pinewood
Proximate analysis
wdry/%Ultimate analysis
wdaf/%V FC A C H N S Oa 85.0 14.4 0.6 51.6 6.2 0.1 0.0 42.1 a: by difference 表 2 无催化剂和不同催化剂作用下生物质加氢热解产生的各类产物产率和气体产物产率以及碳转化率和移入生物油的氧转化率
Table 2 Yields of overall products, individual gases, the carbon conversion and the oxygen conversion by hydropyrolysis of biomass with different catalysts and withot catalyst (NC) at 700 ℃ and 5.0 MPa H2
Products NC Fe(NO3)3 FeSO4 Overall products gas 23.4 ± 1.8 42.9 ± 2.2 21.6 ± 1.6 (daf. biomass basis) w/% water 23.0 ± 1.5 28.1± 1.6 26.2 ± 1.5 bio-oil 34.1 ± 1.8 32.8 ± 1.7 28.6 ± 1.6 chara 15.5 ± 0.1 0.3 ± 0.1 17.9 ± 0.1 sum 96.1 104.0 94.3 Gases CO2 8.7 ± 0.9 12.6 ± 0.8 8.6 ± 0.7 (daf. biomass basis) w/% CO 7.9 ± 0.7 6.7 ± 0.7 5.9 ± 0.6 CH4 4.3 ± 0.5 21.2 ± 0.8 4.7 ± 0.4 C2H6 1.5 ± 0.3 1.6 ± 0.1 1.9 ± 0.2 C2H4 0.1 0.1 0.0 C3H8 0.7 ± 0.1 0.5 ± 0.1 0.4 ± 0.1 C3H6 0.1 0.1 0.1 Carbon conversion (carbon basis) x/% 70.1 97.4 63.0 Oxygen conversion to bio-oil (oxygen basis) /% 25.6 22.1 26.7 a:excluding ash content 表 3 无催化剂和Fe(NO3)3催化剂作用下生物质在不同终温下加氢热解产生的气体和主要生物油产物产率
Table 3 Yields of individual gases and main bio-oil compounds produced by hydropyrolysis of biomass at different temperatures with Fe(NO3)3 and without catalyst (NC) under 5.0 MPa H2
Yield/carbon conversion NC Fe(NO3)3 600 ℃ 650 ℃ 700 ℃ 600 ℃ 650 ℃ 700 ℃ Overall products gas 20.2 21.3 23.4 ± 1.8 25.7 38.1 42.9 ± 2.2 (daf. biomass basis) w/% water 20.4 21.9 23.0 ± 1.5 27.7 28.1 28.1± 1.6 bio-oil 30.6 32.8 34.1 ± 1.8 30.8 31.4 32.8 ± 1.7 chara 23.6 18.0 15.5 ± 0.1 12.8 2.4 0.3± 0.1 sum 94.8 94.1 96.2 97.0 100.0 104.0 Gases (daf. biomass basis)w/% CO2 7.6 8.0 8.7 ± 0.9 11.6 12.5 12.6 ± 0.8 CO 6.7 6.9 7.9 ± 0.7 3.4 5.7 6.7 ± 0.7 CH4 4.0 4.2 4.3 ± 0.5 9.0 17.9 21.2 ± 0.8 C2b 1.3 1.5 1.6 ± 0.3 1.1 1.3 1.7 ± 0.1 C3b 0.7 0.7 0.8 ± 0.1 0.6 0.6 0.6 ± 0.1 Main liquid compounds oxygenates 0.1 0.1 0.0 0.4 0.4 0.8 ± 0.2 (daf. biomass basis)w/% phenols 0.4 0.4 0.4 ± 0.1 0.4 0.4 0.4 ± 0.1 MAHs 0.5 1.1 1.6 ± 0.2 1.0 1.3 2.7 ± 0.3 B & TAHs 0.1 0.5 0.8 ± 0.2 0.2 0.3 0.5 ± 0.1 Carbon conversion (carbon basis)w/% 57.7 66.1 70.0 77.0 93.9 97.4 a: excluding ash content; b: saturated hydrocarbon gases occupied the majority pressure, 5.0 MPa H2 表 4 不同催化剂在不同温度下生物炭的比表面积和孔容
Table 4 Specific surface and pore structure parameters of char samples obtained with different catalysts and with no catalyst (NC)at different temperatures
NC Fe(NO3)3 FeSO4 700 ℃ 400 ℃ 700 ℃ 400 ℃ 700 ℃ Specific surface area A/(m2·g-1) 17.7 3.3 129.6 4.2 3.0 Micropore v/(μL·g-1) 8.2 1.4 56.5 2.0 1.6 Mesoporous v/(μL·g-1) 133.9 19.4 345.7 30.0 16.1 -
[1] 王昶, 李丹, 郝庆兰, 王刚, 宋扬, 李桂菊.粉粒流化床中松木生物质热解特性的研究[J].燃料化学学报, 2012, 40(2):156-163. doi: 10.3969/j.issn.0253-2409.2012.02.005WANG Chang, LI Dan, HE Qin-lan, WANG Gang, SONG Yang, LI Gui-ju. Pyrolysis characteristics of pine biomass in a powder-particle fluidized bed[J]. J Fuel Chem Technol, 2012, 40(2):156-163. doi: 10.3969/j.issn.0253-2409.2012.02.005 [2] BRIDGWATER A V. Review of fast pyrolysis of biomass and product upgrading[J]. Biomass Bioenergy, 2012, 38:68-94. doi: 10.1016/j.biombioe.2011.01.048 [3] HEO H S, PARK H J, PARK Y K, RYU C, SUH D J, SUH Y W, YIM J H, KIM S S. Bio-oil production from fast pyrolysis of waste furniture sawdust in a fluidized bed[J]. Bioresour Technol, 2010, 101:591-596. doi: 10.1016-j.biortech.2009.12.078/ [4] 李缔, 李攀, 王贤华, 邵敬爱, 杨海平, 陈汉平.基于Fe负载的HZSM-5催化热解制备生物油实验研究[J].燃料化学学报, 2016, 44(5):540-547. doi: 10.3969/j.issn.0253-2409.2016.05.005LI Di, LI Pan, WANG Xian-hua, SHAO Jing-ai, YANG Hai-ping, CHEN Han-ping. Experimental study on bio-oil from catalytic pyrolysis on Fe modified HZSM-5[J]. J Fuel Chem Technol, 2016, 44(5):540-547. doi: 10.3969/j.issn.0253-2409.2016.05.005 [5] 张秀梅, 陈冠益, 孟祥梅, 李新禹.催化热解生物质制取富氢气体的研究[J].燃料化学学报, 2004, 32(4):446-449. doi: 10.3969/j.issn.0253-2409.2004.04.012ZHANG Xiu-mei, CHEN Yi-guan, MENG Xiang-mei, LI Xin-yu. Production of hydrogen-rich gas from biomass by catalytic pyrolysis[J]. J Fuel Chem Technol, 2004, 32(4):446-449. doi: 10.3969/j.issn.0253-2409.2004.04.012 [6] WANG K, KIM K H, BROWN R C. Catalytic pyrolysis of individual components of lignocellulosic biomass[J]. Green Chem, 2014, 16:727-735. doi: 10.1039/C3GC41288A [7] KARANJKAR P U, COOLMAN R J, HUBER G W, BLATNIK M T, ALMALKIE S, KOPS S M D B, MOUNTZIARIS T J, CONNER W C. Production of aromatics by catalytic fast pyrolysis of cellulose in a bubbling fluidized bed reactor[J]. Am Inst Chem Eng, 2014, 60:1320-1335. doi: 10.1002/aic.14376 [8] WANG Y M, WANG J. Multifaceted effects of HZSM-5(proton-exchanged zeolite socony mobil-5) on catalytic cracking of pinewood pyrolysis vapor in a two-stage fixed bed reactor[J]. Bioresour Technol, 2016, 214:700-710. doi: 10.1016/j.biortech.2016.05.027 [9] CHANDLER D S, RESENDE F L P. Comparison between catalytic fast pyrolysis and catalytic fast hydropyrolysis for the production of liquid fuels in a fluidized bed reactor[J]. Energy Fuels, 2019, 33:3199-3209. doi: 10.1021/acs.energyfuels.8b03782 [10] WANG K, XU Y, DUAN P, WANG F, XU Z X. Thermo-chemical conversion of scrap tire waste to produce gasoline fuel[J]. Waste Manage, 2019, 86:1-12. doi: 10.1016/j.wasman.2019.01.024 [11] JAN O, MARCHAND R, ANJOS L C A, SEUFITELLI G V S, NIKOLLA E, RESENDE F L P. Hydropyrolysis of Lignin Using Pd/HZSM-5[J]. Energy Fuels, 2015, 29:1793-1800. doi: 10.1021/ef502779s [12] THANGALAZHY-GOPAKUMAR S, ADHIKARI S, GUPTA R B, TU M, TAYLOR S. Production of hydrocarbon fuels from biomass using catalytic pyrolysis under helium and hydrogen environments[J]. Bioresour Technol, 2011, 102:6742-6749. doi: 10.1016/j.biortech.2011.03.104 [13] MEESUK S, CAO J P, SATO K, OGAWA Y, TAKARADA T. The effects of temperature on product yields and composition of bio-oils in hydropyrolysis of rice husk using nickel-loaded brown coal char catalyst[J]. J Anal Appl Pyrolysis, 2012, 94:238-245. doi: 10.1016/j.jaap.2011.12.011 [14] MEESUK S, CAO J-P, SATO K, OGAWA Y, TAKARADA T. Study of catalytic hydropyrolysis of rice husk under nickel-loaded brown coal char[J]. Energy Fuels, 2011, 25:5438-5443. doi: 10.1021/ef201266b [15] ZHENG N, WANG J. Distinctly different performances of two iron-doped charcoals in catalytic hydrocracking of pine wood hydropyrolysis vapor to methane or upgraded bio-oil[J]. Energy Fuels, 2020, 34:546-556. doi: 10.1021/acs.energyfuels.9b03452 [16] ZHENG N, ZHANG J, WANG J. Parametric study of two-stage hydropyrolysis of lignocellulosic biomass for production of gaseous and light aromatic hydrocarbons[J]. Bioresour Technol, 2017, 244:142-150. doi: 10.1016/j.biortech.2017.07.103 [17] MARKER T L, FELIX L G, LINCK M B, ROBERTS M J. Integrated hydropyrolysis and hydroconversion (IH2) for the direct production of gasoline and diesel fuels or blending components from biomass, Part 1:proof of principle testing[J]. Am Inst Chem Eng, 2012, 31:191-199. [18] MARKER T L, FELIX L G, LINCK M B, ROBERTS M J, ORTIZ-TORAL P, WANGEROW J. Integrated hydropyrolysis and hydroconversion (IH2) for the direct production of gasoline and diesel fuels or blending components from biomass, Part 2:Continuous testing[J]. Am Inst Chem Eng, 2013, 33:762-768. http://d.old.wanfangdata.com.cn/NSTLQK/NSTL_QKJJ0225950745/ [19] LUO G, RESENDE F L P. In-situ and ex-situ upgrading of pyrolysis vapors from beetle-killed trees[J]. Fuel, 2016, 166:367-375. doi: 10.1016/j.fuel.2015.10.126 [20] LI B Q, COLETTE B D, RENE C. Catalytic hydropyrolysis by impregnated sulphided Mo catalyst[J]. Fuel, 1991, 70:254-257. doi: 10.1016/0016-2361(91)90161-3 [21] 李文, 李保庆, 孙成功, 尉迟唯, 曹变英.生物质热解、加氢热解及其与煤共热解的热重研究[J].燃料化学学报, 1996, 24(4):341-347. http://www.cqvip.com/Main/Detail.aspx?id=2280284LI Wen, LI Bao-qing, SUN Cheng-gong, WEI Chi-wei, CAO Bian-ying. Study on pyrolysis and hydropyrolysis of biomass and copyrolysis between biomass and coal[J]. J Fuel Chem Technol, 1996, 24(4):341-347. http://www.cqvip.com/Main/Detail.aspx?id=2280284 [22] MEIER D, BERNS J, GRIINWALD C, FAIX O. Analytical pyrolysis and semicontinuous catalytic hydropyrolysis of organocell lignin[J]. J Anal Appl Pyrolysis, 1993, 25:335-347. doi: 10.1016/0165-2370(93)80053-3 [23] DAYTON D C, HLEBAK J, CARPENTER J R, WANG K, MANTE O D, PETERS J E. Biomass hydropyrolysis in a fluidized bed reactor[J]. Energy Fuels, 2016, 30:4879-4887. doi: 10.1021/acs.energyfuels.6b00373 [24] 杨建丽, 李允梅, 阎瑞萍, 崔洪, 刘振宇, 王哲.充州煤的催化加氢及其重质产物的分离和表征[J].煤炭转化, 1998, 21(2):63-67. http://www.cnki.com.cn/Article/CJFDTotal-MTZH199802014.htmYANG Jian-li, LI Yun-mei, YAN Rui-ping, CUI Hong, LIU Zhen-yu, WANG Zhe. Catalytic hydrogenation of YanZhou coal and characterization of the heavy products[J]. Coal Convers, 1998, 21(2):63-67. http://www.cnki.com.cn/Article/CJFDTotal-MTZH199802014.htm [25] STUMMANN M Z, HANSEN A B, HANSEN L P, DAVIDSEN B, RASMUSSEN S B, WIWEL P, GABRIELSEN J, JENSEN P A, JENSEN A D, HØJ M. Catalytic hydropyrolysis of biomass using molybdenum sulfide based catalyst effect of promoters[J]. Energy Fuels, 2019, 33:1302-1313. doi: 10.1021/acs.energyfuels.8b04191 [26] GAMLIEL D P, WILCOX L, VALLA J A. The effects of catalyst properties on the conversion of biomass via catalytic fast hydropyrolysis[J]. Energy Fuels, 2017, 31:679-687. doi: 10.1021/acs.energyfuels.6b02781 [27] VENKATAKRISHNAN V K, DELGASS W N, RIBEIRO F H, AGRAWAL R. Oxygen removal from intact biomass to produce liquid fuel range hydrocarbons via fast-hydropyrolysis and vapor-phase catalytic hydrodeoxygenation[J]. Green Chem, 2015, 17:178-183. doi: 10.1039/C4GC01746C [28] CHANG Z, DUAN P, XU Y. Catalytic hydropyrolysis of microalgae:Influence of operating variables on the formation and composition of bio-oil[J]. Bioresour Technol, 2015, 184:349-354. doi: 10.1016/j.biortech.2014.08.014 [29] VENKATAKRISHNAN V K, DEGENSTEIN J C, SMELTZ A D, DELGASS W N, AGRAWAL R, RIBEIRO F H. High-pressure fast-pyrolysis, fast-hydropyrolysis and catalytic hydrodeoxygenation of cellulose:Production of liquid fuel from biomass[J]. Green Chem, 2014, 16:792-802. doi: 10.1039/c3gc41558a [30] MELLIGAN F, HAYES M H B, KWAPINSKI W, LEAHY J J. A study of hydrogen pressure during hydropyrolysis of Miscanthus x giganteus and online catalytic vapour upgrading with Ni on ZSM-5[J]. J Anal Appl Pyrolysis, 2013, 103:369-377. doi: 10.1016/j.jaap.2013.01.005 [31] MELLIGAN F, HAYES M H B, KWAPINSKI W, LEAHY J J. Hydro-pyrolysis of biomass and online catalytic vapor upgrading with Ni-ZSM-5 and Ni-MCM-41[J]. Energy Fuels, 2012, 26:6080-6090. doi: 10.1021/ef301244h [32] LI L Y, TAKARADA T. Conversion of hot coke oven gas into light fuel gas over Ni/Al2O3 Catalyst[J]. J Chem Eng Jpn, 2006, 39:461-468. doi: 10.1252/jcej.39.461 [33] GAMLIEL D P, BOLLAS G M, VALLA J A. Bifunctional Ni-ZSM-5 catalysts for the pyrolysis and hydropyrolysis of biomass[J]. Energy Technol, 2017, 5:172-182. doi: 10.1002/ente.201600136 [34] DIETRICH M, RONALD A, OSKAR F. Catalytic hydropyrolysis of lignin:Influence of reaction conditions on the formation and composition of liquid products[J]. Bioresour Technol, 1992, 40:171-177. doi: 10.1016/0960-8524(92)90205-C [35] ZHANG J, ZHENG N, WANG J. Two-stage hydrogasification of different rank coals with a focus on relationships between yields of products and coal properties or structures[J]. Appl Energy, 2016, 173:438-447. doi: 10.1016/j.apenergy.2016.04.034 [36] ZHANG J, ZHENG N, WANG J. Comparative investigation of rice husk, thermoplastic bituminous coal and their blends in production of value-added gaseous and liquid products during hydropyrolysis/co-hydropyrolysis[J]. Bioresour Technol, 2018, 268:445-453. doi: 10.1016/j.biortech.2018.08.018 [37] YAN H B, MAO F, WANG J. Thermogravimetric-mass spectrometric characterization of thermal decomposition of lignite with attention to the evolutions of small molecular weight oxygenates[J]. J Anal Appl Pyrolysis, 2020, 146:104781. doi: 10.1016/j.jaap.2020.104781 [38] JIANG Y W, YAN H B, GUO Q H, WANG F C, WANG J. Multiple synergistic effects exerted by coexisting sodium and iron on catalytic steam gasification of coal char[J]. Fuel Process Technol, 2019, 191:1-10. doi: 10.1016/j.fuproc.2019.03.017 [39] ZHONG M, ZHAO Y, ZHAI J R, JIN J L, HU H Q, BAI Z Q, LI W. Effects of nickel additives with different anions on the structure and pyrolysis behavior of Hefeng coal[J]. Fuel Process Technol, 2019, 193:273-281. doi: 10.1016/j.fuproc.2019.05.030 -





 下载:
下载: