Preparation of MoS2/TixOy catalysts via a one-pot solvothermal method for electrocatalytic water splitting to produce hydrogen
-
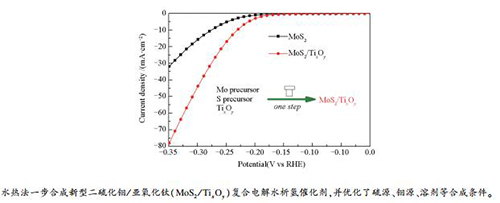
摘要: 通过水热法一步合成了系列二硫化钼/亚氧化钛(MoS2/TixOy)复合催化剂,研究了溶剂、硫源和钼源等合成条件对所合成的催化剂电催化析氢活性的影响以及亚氧化钛的作用。结果表明,溶剂、硫源、钼源、亚氧化钛等因素都对催化剂的结构和电解水析氢性能有重要影响。溶剂水、水解可产生铵根离子的硫源和钼源以及亚氧化钛的加入有利于获得具有高析氢活性的催化剂。其中,以水为溶剂、硫代乙酰胺为硫源、钼酸铵为钼源得到的催化剂析氢活性最高,电解水析氢测试中达到10 mA/cm2电流密度时需要的过电势仅为280 mV。Abstract: A series of MoS2/TixOy catalysts were prepared by a one pot solvothermal synthesis method and the effects of solvent, sulfur source, molybdenum source and titanium subdioxide conductive agent on the electrocatalytic activity of MoS2/TixOy in hydrogen evolution from water splitting were investigated. The results showed that the crystal structure of MoS2/TixOy catalyst as well as its catalytic performance is greatly influenced by the solvent, sulfur source, molybdenum source and titanium subdioxide conductive agent. Water, sulfur and molybdenum sources which can produce ammonium ions via hydrolysis, and the conductive agents are beneficial to improving the hydrogen evolution activity of the MoS2/TixOy catalyst in water splitting. In particular, with water as the solvent, thioacetamide and ammonium molybdate as the sulfur and molybdenum sources, respectively, the MoS2/TixOy catalyst with the highest hydrogen evolution activity was obtained; it needs only 280 mV overpotential to reach 10 mA/cm2 current density in the electrolysis of water.
-
Key words:
- hydrogen evolution /
- MoS2/TixOy /
- solvothermal synthesis /
- water splitting
-
图 1 钼酸钠+硫代乙酰胺+TixOy在不同溶剂下合成得到的催化剂、TixOy以及2H-MoS2 (JCPDS 37-1492)和Mo15S19(JCPDS 40-0936)XRD谱图
Figure 1 XRD patterns of the MoS2/TixOy catalysts synthesized with sodium molybdate + thioacetamide + TixOy in different solvents, in comparison with TixOy, 2H-MoS2 (JCPDS 37-1492) and Mo15S19 (JCPDS 40-0936)
-
[1] PACALA S, SOCOLOW R. Stabilization wedges:Solving the climate problem for the next 50 years with current technologies[J]. Science, 2004, 305(5686):968-972. doi: 10.1126/science.1100103 [2] GONG M, WANG D Y, CHEN C C, HWANG B J, DAI H J. A mini review on nickel-based electrocatalysts for alkaline hydrogen evolution reaction[J]. Nano Res, 2016, 9(1):28-46. doi: 10.1007/s12274-015-0965-x [3] LU Q, YU Y, MA Q, CHEN B, ZHANG H. 2D Transition-metal-dichalcogenide-nanosheet-based composites for photocatalytic and electrocatalytic hydrogen evolution reactions[J]. Adv Mater, 2016, 28(10):1917-1933. doi: 10.1002/adma.201503270 [4] MAHMOOD J, LI F, JUNG S M, OKYAY M S, AHMAD I, KIM S J, PARK N, JEONG H Y, BAEK J B. An efficient and pH-universal ruthenium-based catalyst for the hydrogen evolution reaction[J]. Nat Nanotechnol, 2017, 12(5):441-446. doi: 10.1038/nnano.2016.304 [5] STAMENKOVIC V R, MUN B S, ARENZ M, MAYRHOFER K J J, LUCAS C A, WANG G F, ROSS P N, MARKOVIC N M. Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces[J]. Nat Mater, 2007, 6(3):241-247. doi: 10.1038/nmat1840 [6] HE Q, WAN Y, JIANG H, WU C, SUN Z, CHEN S, ZHOU Y, CHEN H, LIU D, HALEEM Y, GE B, WU X, SONG L. High-metallic-phase-concentration Mo1-xWxS2 nanosheets with expanded interlayers as efficient electrocatalysts[J]. Nano Res, 2018, 11(3):1687-1698. doi: 10.1007/s12274-017-1786-x [7] JARAMILLO T F, JØRGENSEN K P, BONDE J, NIELSEN J H, HORCH S, CHORKENDORFF I. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts[J]. Science, 2007, 317(5834):100-102. doi: 10.1126/science.1141483 [8] HINNEMANN B, MOSES P G, BONDE J, JØRGENSEN K P, NIELSEN J H, HORCH S CHORKENDORFF I, NØRSKOV J K. Biomimetic hydrogen evolution:MoS2 nanoparticles as catalyst for hydrogen evolution[J]. J Am Chem Soc, 2005, 127(15):5308-5309. doi: 10.1021/ja0504690 [9] DING Q, SONG B, XU P, JIN S. Efficient electrocatalytic and photoelectrochemical hydrogen generation using MoS2 and related compounds[J]. Chem, 2016, 1(5):699-726. doi: 10.1016/j.chempr.2016.10.007 [10] JAYABAL S, SARANYA G, WU J, LIU Y, GENG D, MENG X. Understanding the high-electrocatalytic performance of two-dimensional MoS2 nanosheets and their composite materials[J]. J Mater Chem A, 2017, 5(47):24540-24563. doi: 10.1039/C7TA08327K [11] LV Z, MAHMOOD N, TAHIR M, PAN L, ZHANG X, ZOU J. Fabrication of zero to three dimensional nanostructured molybdenum sulfides and their electrochemical and photocatalytic applications[J]. Nanoscale, 2016, 8(43):18250-18269. doi: 10.1039/C6NR06836G [12] CHHOWALLA M, SHIN H S, EDA G, LI L, LOH K, ZHANG H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets[J]. Nat Chem, 2013, 5(4):263-275. doi: 10.1038/nchem.1589 [13] LV R T, ROBINSON J A, SCHAAK R E, SUN D, SUN Y F, MALLOUK T E, TERRONES M. Transition metal dichalcogenides and beyond:Synthesis, properties, and applications of single- and few-layer nanosheets[J]. ACC Chem Res, 2015, 48(1):56-64. doi: 10.1021/ar5002846 [14] LIU Q, LI X L, XIAO Z R, ZHOU Y, CHEN H P, KHALIL A, XIANG T, XU J Q, CHU W S, WU X J, YANG J L, WANG C M, XIONG Y J, JIN C H, AJAYAN P M, SONG L. Stable metallic 1T-WS2 nanoribbons intercalated with ammonia ions:The correlation between structure and electrical/optical properties[J]. Adv Mater, 2015, 27(33):4837-4844. doi: 10.1002/adma.201502134 [15] XUE N, DIAO P. Composite of few-layered MoS2 grown on carbon black:Tuning the ratio of terminal to total sulfur in MoS2 for hydrogen evolution reaction[J]. J Phys Chem C, 2017, 121(27):14413-14425. doi: 10.1021/acs.jpcc.7b02522 [16] LI Y G, WANG H L, XIE L M, LIANG Y Y, HONG G S, DAI H J. MoS2 nanoparticles grown on graphene:An advanced catalyst for the hydrogen evolution reaction[J]. J Am Chem Soc, 2011, 133(19):7296-7299. doi: 10.1021/ja201269b [17] TANG S B, WU W H, ZHANG S Y, YE D N, ZHONG P, LI X K, LIU L X, LI Y F. Tuning the activity of the inert MoS2 surface via graphene oxide support doping towards chemical functionalization and hydrogen evolution:A density functional study[J]. Phys Chem Chem Phys, 2018, 20(3):1861-1871. doi: 10.1039/C7CP06636H [18] DUMA A D, WU Y C, SU W N, PAN C J, TSAI M C, CHEN H M, LEE J F, SHEU H S, HO V T T, HWANG B J. In situ confined synthesis of Ti4O7 supported platinum electrocatalysts with enhanced activity and stability for the oxygen reduction reaction[J]. ChemCatChem, 2018, 10(5):1155-1165. doi: 10.1002/cctc.201701503 [19] IBRAHIM K B, SU W N, TSAI M C, CHALA S A, KAHSAY A W, YEH M H, CHEN H M, DUMA A D, DAI H J, HWANG B J. Robust and conductive magneli phase Ti4O7 decorated on 3D-nanoflower NiRu-LDH as high-performance oxygen reduction electrocatalyst[J]. Nano Energy, 2018, 47:309-315. doi: 10.1016/j.nanoen.2018.03.017 [20] IOROI T, SENOH H, YAMAZAKI S I, SIROMA Z, FUJIWARA N, YASUDA K. Stability of corrosion-resistant magneli-phase Ti4O7-supported PEMFC catalysts at high potentials[J]. J Electrochem Soc, 2008, 155(4):B321-B326. doi: 10.1149/1.2833310 [21] LIU Q, LI X L, HE Q, KHALIL A, LIU D B, XIANG T, WU X J, SONG L. Gram-scale aqueous synthesis of stable few-layered 1T-MoS2:Applications for visible-light-driven photocatalytic hydrogen evolution[J]. Small, 2015, 11(41):5556-5564. doi: 10.1002/smll.201501822 [22] LIU Y, WU K, GUO X L, WANG W Y, YANG Y Q. A comparison of MoS2 catalysts hydrothermally synthesized from different sulfur precursors in their morphology and hydrodeoxygenation activity[J]. J Fuel Chem Technol, 2018, 46(5):34-41. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rlhxxb201805004 [23] YIN Y, HAN J C, ZHANG Y M, ZHANG X H, XU P, YUAN Q, SAMAD L, WANG X J, WANG Y, ZHANG Z H, ZHANG P, CAO X Z, SONG B, JIN S. Contributions of phase, sulfur vacancies, and edges to the hydrogen evolution reaction catalytic activity of porous molybdenum disulfide nanosheets[J]. J Am Chem Soc, 2016, 138(25):7965-7972. doi: 10.1021/jacs.6b03714 -





 下载:
下载: