One-step preparation of mesoporous carbon containing tungsten and its desulfurization performance
-
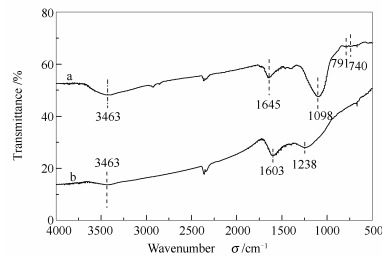
摘要: 以钨酸钠为钨源,以乙二胺四乙酸二钠为碳源经过高温煅烧制备了含W的介孔碳材料,采用XRD、SEM、FT-IR、BET对含钨的介孔碳材料进行表征。结果表明,煅烧后介孔碳材料的表面形成了粒状含有结晶水的氧化钨(WO3·H2O)。相比于纯的介孔碳材料,含钨介孔碳材料的总比表面积减小。以含W介孔碳材料为催化剂,H2O2作为氧化剂,1-丁基-3-甲基咪唑氟硼酸盐([BMIM][BF4])离子液体作为萃取剂,组成萃取-催化氧化脱硫体系(ECODS)并研究其对模拟油中二苯并噻吩脱除效果。考察了氧化钨负载量、反应温度、H2O2加入量、催化剂用量、离子液体用量以及不同类型硫化物对二苯并噻吩脱除的影响。在最佳反应条件下,催化剂对二苯并噻吩(DBT)、4,6-二甲基二苯并噻吩(4,6-DMDBT)、苯并噻吩(BT)、噻吩(TH)和真实汽油的脱除率分别达到98.6%、65.6%、61.2%、57.8%和64.3%。催化剂回收利用五次之后脱硫率略有降低,仍高达95.2%。Abstract: A mesoporous carbon containing tungsten was prepared using Na2WO4 and EDTA-2Na as the source of tungsten and carbon and characterized by XRD, FT-IR, SEM and BET. The results show that the tungsten oxide with crystal water (WO3·H2O) is formed on the surface of mesoporous carbon. Compared with pure mesoporous carbon, the total surface area of mesoporous carbon containing tungsten decreases. An extraction-catalytic oxidation desulfurization (ECODS) system was constructed using mesoporous carbon containing tungsten as catalyst, H2O2 as oxidant and ionic liquids (ILs) 1-butyl-3-methyl imidazolium tetrafluoroborate ([BMIM] [BF4]) as extraction agent. The effects of tungsten oxide loading, reaction temperature, the amount of H2O2, the amount of catalyst and ILs dosage and different types of sulfur compounds on the removal of DBT was studied. Under the optimal conditions, the removal rate of DBT reaches 98.6% for DBT, 65.6% for 4, 6-DMDBT, 61.2% for BT, 57.8% for TH and 64.3% for actual gasoline, respectively. The desulfurization rate is slightly decreased when the catalyst is reused five times.
-
Key words:
- WO3·H2O /
- catalytic oxidation desulfurization /
- extraction /
- ILs
-
表 1 不同负载量催化剂的比表面积及孔结构
Table 1 BET surface area and pore structure of catalysts under different loading conditions
Catalyst Specific area A/(m2·g-1) Pore volume v/(cm3·g-1) Pore size d/nm MC 460.19 0.130 2.93 W-5%/MC 28.99 0.042 4.03 W-10%/MC 50.77 0.034 3.21 W-20%/MC 138.35 0.044 3.25 表 2 离子液体用量对脱硫效果的影响
Table 2 Effect of ILs amount on desulfurization efficiency
ILs amount V/mL S-removal η/% ILs amount V/mL S-removal η/% 0 24.4 0.5 99 0.2 93 1.0 98.4 0.25 98.6 1.5 98 reaction conditions:V(model oil)=5 mL, t=70 ℃, V(H2O2)=0.2 mL, m(catalyst)=0.02 g 表 3 不同硫化物的脱除
Table 3 Removal effect of different sulfides
Desulfurization system S-removal η /% 4, 6-DMDBT DBT BT TH Catalyst +ILs+H2O2 65.6 98.6 61.2 57.8 ILs+H2O2 14.5 24.4 12.7 8.2 Catalyst +H2O2 9.8 12.2 8.6 7.3 H2O2 < 2 < 2 < 2 < 2 表 4 催化剂和离子液体的循环使用对脱硫效果的影响
Table 4 Effect of the recycling catalyst and ILs on desulfurization efficiency
Cycle number Desulfurization rate η /% Cycle times Desulfurization rate η /% 0 98.6 3 97.5 1 98.3 4 96.4 2 97.9 5 95.2 reaction conditions:V(model oil)=5 mL, t=70 ℃, V(H2O2)=0.2 mL, m(catalyst)=0.02 g, V(ILs)=0.25 mL -
[1] YU F L, LIU C Y, XIE P H, XIE C X, YU S T. Oxidative-extractive deep desulfurization of gasoline by functionalized heteropoly acid catalysts[J]. RSC Adv, 2015, 5(104):85540-85546. doi: 10.1039/C5RA16013H [2] GB 17930-2013, Gasoline for motor vehicles[S]. [3] ZHANG H, GAO J, MENG H, LI C X. Removal of thiophenic sulfurs using an extractive oxidative desulfurization process with three new phosphotungstate catalysts[J]. Ind Eng Chem Res, 2012, 51:6658-6665. doi: 10.1021/ie3004545 [4] ZHU W S, WANG C, LI H P, WU P W, XUN S H, JIANG W, CHEN Z G, ZHAO Z, LI H M. One-pot extraction combined with metal-free photochemical aerobic oxidative desulfurization in deep eutectic solvent[J]. Green Chem, 2015, 17(4):2464-2472. doi: 10.1039/C4GC02425G [5] CAMPOS-MARTIN J M, CAPEL-SANCHEZ M C, FIERRO J L G. Highly efficient deep desulfurization of fuels by chemical oxidation[J]. Green Chem, 2004, 6(11):557-562. doi: 10.1039/b409882j [6] AGGARWAL S, KARIMI I A, LEE D Y. Reconstruction of a genome-scale metabolic network of Rhodococcus erythropolis for desulfurization studies[J]. Mol BioSyst, 2011, 7(11):3122-3131. doi: 10.1039/c1mb05201b [7] HUANG C P, CHEN B H, ZHANG J, LIU Z C, LI Y. Desulfurization of gasoline by extraction with new ionic liquids[J]. Energy Fuels, 2004, 18(6):1862-1864. doi: 10.1021/ef049879k [8] ZHAO K, CHENG Y, LIU H Y, YANG C P, QIU L, ZENG G M, HE H J. Extractive desulfurization of dibenzothiophene by a mixed extractant of N, N-dimethylacetamide, N, Ndimethylformamide and tetramethylene sulfone:Optimization by Box-Behnken design[J]. RSC Adv, 2015, 5(81):66013-66023. doi: 10.1039/C5RA12305D [9] LENK K Y, SUN Y Y, ZHANG X, XU W. Ti-modified hierarchical mordenite as highly active catalyst for oxidative desulfurization of dibenzothiophene[J]. Fuel, 2016, 174:9-16. doi: 10.1016/j.fuel.2016.01.070 [10] ZHOU M D, MENG W Y, LI Y, WANG Q, LI X B, ZANG S L. Extractive and catalytic oxidative desulfurization of gasoline by methyltrioxorhenium in ionic liquids[J]. Energy Fuels, 2013, 28(1):516-521. [11] CHAMACK M, MAHJOUB A R. Synthesis and characterization of supported Cs2H[PW4Mo8O40] on iron oxide@mesoporous silica particles:Promising catalyst for oxidative desulfurization process[J]. Catal Lett, 2016, 146(6):1050-1058. doi: 10.1007/s10562-016-1731-8 [12] SHIRANI M, SEMNANI A, HABIBOLLAHI S, HADDADI H. Synthesis and application of magnetic NaY zeolite composite immobilized with ionic liquid for adsorption desulfurization of fuel using response surface methodology[J]. J Porous Mater, 2016, 23(3):701-712. doi: 10.1007/s10934-016-0125-z [13] JAIN N, KUMAR A, CHAUHAN S. Chemical and biochemical transformations in ionic liquids[J]. Tetrahedron, 2005, 61(5):1015-1060. doi: 10.1016/j.tet.2004.10.070 [14] VAN R F, SHELDON R A. Biocatalysis in ionic liquids[J]. Chem Rev, 2007, 107(6):2757-2785. doi: 10.1021/cr050946x [15] SONG C E, ROH E J. Practical method to recycle a chiral (salen) Mn epoxidation catalyst by using an ionic liquid[J]. Chem Commun, 2000, 31(35):837-838. https://www.researchgate.net/publication/237771721_Practical_Method_to_Recycle_a_Chiral_SalenMn_Epoxidation_Catalyst_by_Using_an_Ionic_Liquid [16] BÖSMANN A, DATSEVICH L, JESS A. Deep desulfurization of diesel fuel by extraction with ionic liquids[J]. Petrol Sci Technol, 2008, 26(9):973-982. doi: 10.1080/10916460600695496 [17] ZHAO D S, WANG J L, ZHOU E P. Oxidative desulfurization of diesel fuel using a Brønsted acid room temperature ionic liquid in the presence of H2O2[J]. Green Chem, 2007, 9(11):1219-1222. doi: 10.1039/b706574d [18] WU Z Y, IQBAL Z, WANG X Q. Metal-free, carbon-based catalysts for oxygen reduction reactions[J]. Chem Sci Eng, 2015, 9(3):280-294. https://www.researchgate.net/publication/282890244_Metal-Free_Carbon-Based_Catalysts_for_Oxygen_Reduction_Reactions [19] LI X, ZHU H W, WANG A J, CHEN Y Y. Oxidative desulfurization of dibenzothiophene over tungsten oxides supported on SiO2 and γ-Al2O3[J]. Chem Lett, 2013, 42(1):8-10. doi: 10.1246/cl.2013.8 [20] LI X C, HUANG S X, XU Q R, YANG Y F. Preparation of WO3-SBA-15 mesoporous molecular sieve and its performance as an oxidative desulfurization catalyst[J]. Transit Metal Chem, 2009, 34(8):943-947. doi: 10.1007/s11243-009-9285-x [21] TORRES-GARCIA E, CANIZAL G, VELUMANI S, RAMIREZ-VERDUZCO L, MURRIETA-GUEVARA F, ASCENCIO J. Influence of surface phenomena in oxidative desulfurization with WOx/ZrO2 catalysts[J]. Appl Phys A:Mater, 2004, 79(8):2037-2040. doi: 10.1007/s00339-004-2668-0 [22] ZHAO R X, LI X P, SU J X, GAO X H. Preparation of WO3/g-C3N4 composites and their application in oxidative desulfurization[J]. Appl Surf Sci, 2017, 392:810-816. doi: 10.1016/j.apsusc.2016.08.120 [23] KATSUMATA H, TACHI Y, SUZUKI T, KANECO S. Z-scheme photocatalytic hydrogen production over WO3/g-C3N4 composite photocatalysts[J]. Rsc Adv, 2014, 4(41):21405-21409. doi: 10.1039/c4ra02511c [24] SHI Y W, LIU G Z, WANG L, ZHANG X W. Efficient adsorptive removal of dibenzothiophene from model fuel over heteroatom-doped porous carbons by carbonization of an organic salt[J]. Chem Eng J, 2015, 259:771-778. doi: 10.1016/j.cej.2014.08.054 [25] DING J, LIU Q Q, ZHANG Z Y, LIU X, ZHAO J Q, CHENG S B, ZONG B N, DAI W L. Carbon nitride nanosheets decorated with WO3 nanorods:Ultrasonic-assisted facile synthesis and catalytic application in the green manufacture of dialdehydes[J]. Appl Catal B:Environ, 2015, 165:511-518. doi: 10.1016/j.apcatb.2014.10.037 [26] QIU J H, WANG G H, ZHANG Y Q, ZENG D L, CHEN Y. Direct synthesis of mesoporous H3PMo12O40/SiO2 and its catalytic performance in oxidative desulfurization of fuel oil[J]. Fuel, 2015, 147:195-202. doi: 10.1016/j.fuel.2015.01.064 [27] ZHUANG J Z, HU B, TAN J J, JIN X Y. Deep oxidative desulfurization of dibenzothiophene with molybdovanadophosphoric heteropolyacid-based catalysts[J]. Transit Metal Chem, 2014, 39(2):213-220. doi: 10.1007/s11243-013-9792-7 [28] 谢东, 何其慧, 苏阳洋, 王童薇, 许仁富, 胡柏星. MCM-41分子筛负载亚硒核过氧钨酸盐催化剂催化二苯并噻吩氧化脱硫[J].催化学报, 2015, 36(8):1205-1213. doi: 10.1016/S1872-2067(15)60897-XXIE Dong, HE Qi-hui, SU Yang-yang, WANG Tong-wei, XU Ren-fu, HU Bai-xing. Oxidative desulfurization of dibenzothiophene catalyzed by peroxotungstate on functionalized MCM-41 materials using hydrogen peroxide as oxidant[J]. Chin J Catal, 2015, 36(8):1205-1213. doi: 10.1016/S1872-2067(15)60897-X [29] ZHENG D, ZHU W S, XUN S H, ZHOU M M, ZHANG M, JIANG W, QIN Y J, LI H M. Deep oxidative desulfurization of dibenzothiophene using low-temperature-mediated titanium dioxide catalyst in ionic liquids[J]. Fuel, 2015, 159(6):446-453. https://www.researchgate.net/profile/Wenshuai_Zhu/publication/281720529_Deep_oxidative_desulfurization_of_dibenzothiophene_using_lowerature-mediated_titanium_dioxide_catalyst_in_ionic_liquids/links/562086fe08aea35f267e19ac.pdf [30] CEDEÑO-CAERO L, GOMEZ-BERNAL H, FRAUSTRO-CUEVAS A. Oxidative desulfurization of synthetic diesel using supported catalysts:Part Ⅲ. Support effect on vanadium-based catalysts[J]. Catal Today, 2008, 133(1):244-254. https://www.researchgate.net/publication/229175168_Oxidative_desulfurization_of_synthetic_diesel_using_supported_catalysts_Part_III_Support_effect_on_vanadium-based_catalysts [31] DAI B L, WU P W, ZHU W S, CHAO Y H, SUN J, XIONG J, JAING W, LI H M. Heterogenization of homogenous oxidative desulfurization reaction on graphene-like boron nitride with a peroxomolybdate ionic liquid[J]. RSC Adv, 2015, 6(1):140-147. [32] OTSUKI S, NONAKA T, TAKASHIMA N, QIAN W H, ISHIHARA A, IMAI T, KABE T. Oxidative desulfurization of light gas oil and vacuum gas oil by oxidation and solvent extraction[J]. Energy Fuels, 2000, 14(6):1232-1239. doi: 10.1021/ef000096i [33] LO W H, YANG H Y, WEI G T. One-pot desulfurization of light oils by chemical oxidation and solvent extraction with room temperature ionic liquids[J]. Green Chem, 2003, 5(5):639-642. doi: 10.1039/b305993f [34] 李宇慧, 冯丽娟, 王景刚, 徐康文, 李春虎. MoO3/Al2O3介孔催化剂在柴油氧化脱硫中的应用[J].石油学报 (石油加工), 2011, 27(6):878-883. http://www.cnki.com.cn/Article/CJFDTotal-SXJG201106007.htmLI Yu-hui, FENG Li-juan, WANG Jing-gang, XU Kang-wen, LI Chun-hu. Oxidative desulfurization of diesel oil by mesoporous catalyst MoO3/Al2O3[J]. Acta Pet Sin (Pet Process Sect), 2011, 27(6):878-883. http://www.cnki.com.cn/Article/CJFDTotal-SXJG201106007.htm [35] ESSER J, WASSERSCHEID P, JESS A. Deep desulfurization of oil refinery streams by extraction with ionic liquids[J]. Green Chem, 2004, 6(7):316-322. doi: 10.1039/B407028C [36] ZHAO D S, SUN Z M, LI F T, LIU R, SHAN H D. Oxidative desulfurization of thiophene catalyzed by (C4H9)4NBr·2C6H11NO coordinated ionic liquid[J]. Energy Fuels, 2008, 22(5):3065-3069. doi: 10.1021/ef800162w [37] TANG X D, HU T, LI J J, WANG F, QING D Y. Deep desulfurization of condensate gasoline by electrochemical oxidation and solvent extraction[J]. RSC Adv, 2015, 5(66):53455-53461. doi: 10.1039/C5RA06851G [38] SHIRAISHI Y, TACHIBANA K, HIRAI T, KOMASAWA I. Desulfurization and denitrogenation process for light oils based on chemical oxidation followed by liquid-liquid extraction[J]. Ind Eng Chem Res, 2002, 41(17):4362-4375. doi: 10.1021/ie010618x [39] DONG Y, NIE Y, ZHOU Q. Highly efficient oxidative desulfurization of fuels by Lewis acidic ionic liquids based on iron chloride[J]. Chem Eng Technol, 2013, 36(3):435-442. doi: 10.1002/ceat.v36.3 [40] USUI Y, SATO K. A green method of adipic acid synthesis:Organic solvent-and halide-free oxidation of cycloalkanones with 30% hydrogen peroxide[J]. Green Chem, 2003, 5(4):373-375. doi: 10.1039/b305847f -





 下载:
下载: