Performance of pentaethylenehexamine modified MIL-101(Cr) metal-organic framework in CO2 adsorption
-
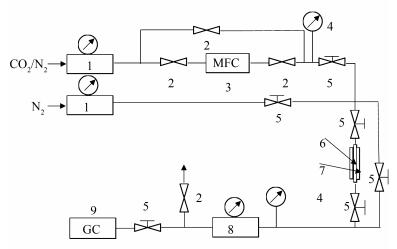
摘要: 采用溶剂热法合成金属有机骨架材料MIL-101(Cr),用回流法将五乙烯六胺(PEHA)负载到MIL-101(Cr)孔道中的不饱和金属位点上,使用扫描电镜、粉末X射线衍射、氮气物理吸附、元素分析和傅里叶变换红外光谱等表征手段考察材料的结构和形貌,测试氨基改性前后的MIL-101(Cr)在25℃、不同压力下对CO2的吸附效果。结果表明,负载0.24 mL五乙烯六胺后的MIL-101(Cr)对CO2的吸附效果最好,在25℃、常压下对CO2的饱和吸附量可达58.944 mg/g,相比未负载五乙烯六胺的MIL-101(Cr)吸附量(CO2饱和吸附量为44.208 mg/g)增加了33%。随着吸附压力的增加,MIL-101(Cr)和0.24PEHA-MIL-101(Cr)对CO2的饱和吸附量逐渐增加,当吸附压力为1.1 MPa时,两者的吸附量分别为1 147.59和1 256.74 mg/g,表明该类材料在高压下对CO2有着良好的吸附效果。
-
关键词:
- 金属有机骨架 (MOF) /
- MIL-101(Cr) /
- 五乙烯六胺 /
- 二氧化碳 /
- 吸附
Abstract: The metal-organic framework MIL-101(Cr) was synthesized via hydrothermal process and then modified with pentaethylenehexamine (PEHA) through refluxing in ethanol. Various measures such as SEM, XRD, N2 sorption, Elemental analysis and FT-IR were used to characterize the structure, morphology and properties of PEHA-grafted MIL-101(Cr). Meanwhile, the performance of PEHA-grafted MIL-101(Cr) in CO2 adsorption was investigated under 25℃. The results illustrate that the loading of PEHA in MIL-101(Cr) can conspicuously enhance the CO2 adsorption capacity. PEHA-grafted MIL-101(Cr) with a PEHA loading of 0.24 mL exhibits the highest capacity for CO2 adsorption; the adsorption capacity reaches 58.944 mg/g at 25℃ and atmospheric pressure, which is 33% higher than that of the unmodified MIL-101(Cr) (44.208 mg/g). In addition, the CO2 adsorption capacities on both MIL-101(Cr) and PEHA-MIL-101(Cr) are greatly enhanced by increasing pressure, reaching 1 147.59 and 1 256.74 mg/g at 1.1 MPa, respectively. These results suggest that PEHA-modified MIL-101(Cr) could be a good candidate adsorbent for CO2 capture at high pressure.-
Key words:
- metal-organic frameworks /
- MIL-101(Cr) /
- pentaethylenehexamine /
- CO2 /
- absorption
-
表 1 样品的比表面积和孔容
Table 1 Surface area and pore volume of various PEHA-MIL-101(Cr) samples
Sample ABET
/(m2·g-1)Pore volume
v/(cm3·g-1)MIL-101 3 625 1.809 0.12PEHA-MIL-101 2 503 1.604 0.24PEHA-MIL-101 1 791 1.385 0.36PEHA-MIL-101 1 246 1.131 表 2 样品的元素分析
Table 2 Results of various PEHA-MIL-101(Cr) samples
Sample Elemental content w/% N C H MIL-101 1.488 36.04 2.740 0.12PEHA-MIL-101 4.585 38.24 3.401 0.24PEHA-MIL-101 8.250 41.66 4.230 0.36PEHA-MIL-101 8.107 42.04 4.254 -
[1] SUMIDA K, ROGOW D L, MASON J A, MCDONALD T M, BLOCH E D, HERM Z R, BAE T H, LONG J R. Carbon dioxide capture in metal-organic frameworks[J]. Chem Rev, 2011, 112(2):724-781. [2] YANG H, XU Z, FAN M, GUPTA R, SLIMANE R B, BLAND A E, WRIGHT I. Progress in carbon dioxide separation and capture:A review[J]. J Environ Sci, 2008, 20(1):14-27. doi: 10.1016/S1001-0742(08)60002-9 [3] CHAKMA A, MEHROTRA A K, NIELSEN B. Comparison of chemical solvents for mitigating CO2, emissions from coal-fired power plants[J]. Heat Recovery Syst CHP, 1995, 15(2):231-240. doi: 10.1016/0890-4332(95)90030-6 [4] WANG X, AKHMEDOV N G, HOPKINSON D, HOFFMAN J, DUAN Y, EGBEBI A, RESNIK K, LI B. Phase change amino acid salt separates into CO2-rich and CO2-lean phases upon interacting with CO2[J]. Appl Energy, 2016, 161:41-47. doi: 10.1016/j.apenergy.2015.09.094 [5] 杨刚胜, 曾淦宁, 赵强, 陈徐, 陈盛积, 艾宁.负载型氨基酸离子液体的制备及其对二氧化碳的吸附性能[J].燃料化学学报, 2016, 44(1):106-112. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18768.shtmlYANG Gang-sheng, ZENG Gan-ning, ZHAO Qiang, CHEN Xu, CHEN Sheng-ji, AI Ning. Preparation of silica gel supported amino acid ionic liquids and their performance capacity towards carbon dioxide[J]. J Fuel Chem Technol, 2016, 44(1):106-112. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18768.shtml [6] 凌凡, 张忠孝, 樊俊杰, 安海泉.膜分离法、化学吸收法以及联合法分离CO2/CH4试验比较[J].动力工程学报, 2015, 35(3):245-250. http://www.cqvip.com/QK/95606X/201503/665070686.htmlLIN Fan, ZHANG Zhong-xiao, FAN Jun-jie, AN Hai-quan. Experimental study on CO2/CH4 separation respectively by membrane, chemical and the combine method[J]. Chin J Power Eng, 2015, 35(3):245-250. http://www.cqvip.com/QK/95606X/201503/665070686.html [7] BATES E D, MAYTON R D, NTAI I, DAVIS J H. CO2 capture by a task-specific ionic liquid[J]. J Am Chem Soc, 2002, 124(6):926-927. doi: 10.1021/ja017593d [8] CEJKA J, CORMA A, ZONES S. Zeolites and Catalysis:Synthesis, Reactions and Applications[M]. Hoboken:John Wiley & Sons, 2010. [9] CAVENATI S, GRANDE C A, RODRIGUES A E. Adsorption equilibrium of methane, carbon dioxide, and nitrogen on zeolite 13X at high pressures[J]. J Chem Eng Data, 2004, 49(4):1095-1101. doi: 10.1021/je0498917 [10] CHUE K, KIM J, YOO Y, CHO S, YANG R. Comparison of activated carbon and zeolite 13X for CO2 recovery from flue gas by pressure swing adsorption[J]. Ind Eng Chem Res, 2002, 342(2):591-598. https://www.researchgate.net/publication/231367110_Comparison_of_Activated_Carbon_and_Zeolite_13X_for_CO2_Recovery_from_Flue_Gas_by_Pressure_Swing_Adsorption [11] 张所瀛, 刘红, 刘朋飞, 吴培培, 杨祝红, 阳庆元, 陆小华.金属有机骨架材料在CO2/CH4吸附分离中的研究进展[J].化工学报, 2014, 65(5):1563-1570. http://d.g.wanfangdata.com.cn/Periodical_hgxb201405002.aspxZHANG Suo-ying, LIU Hong, LIU Peng-fei, WU Pei-pei, YANG Zhu-hong, YANG Qing-yuan, LU Xiao-hua. Progress of adsorption-based CO2/CH4 separation by metal organic frameworks[J]. J Chem Ind Eng, 2014, 65(5):1563-1570. http://d.g.wanfangdata.com.cn/Periodical_hgxb201405002.aspx [12] MILLWARD A R, YAGHI O M. Metal-organic frameworks with exceptionally high capacity for storage of carbon dioxide at room temperature[J]. J Am Chem Soc, 2005, 127(51):17998-17999. doi: 10.1021/ja0570032 [13] ZIAEE A, CHOVAN D, LUSI M, PERRY IV J J, ZAWOROTKO M J, TOFAIL S A. Theoretical optimization of pore size and chemistry in SIFSIX-3-M hybrid ultramicroporous materials[J]. Cryst Growth Des, 2016, 16(7):3890-3897. doi: 10.1021/acs.cgd.6b00453 [14] BRITT D, FURUKAWA H, WANG B, GLOVER T G, YAGHI O M. Highly efficient separation of carbon dioxide by a metal-organic framework replete with open metal sites[J]. Proc Natl Acad Sci U S A, 2009, 106(49):20637-20640. doi: 10.1073/pnas.0909718106 [15] FÉREY G, MELLOT-DRAZNIEKS C, SERRE C, MILLANGE F, DUTOUR J, SURBLÉ S, MARGIOLAKI I. A chromium terephthalate-based solid with unusually large pore volumes and surface area.[J]. Science, 2005, 309(5743):2040-2042. doi: 10.1126/science.1116275 [16] HWANG Y K, HONG D Y, CHANG J S, JHUNG S H, SEO Y K, KIM J, VIMONT A, DATURI M, SERRE C, FÉREY G. Amine grafting on coordinatively unsaturated metal centers of MOFs:Consequences for catalysis and metal encapsulation[J]. Angew Chem Int Ed Eng, 2008, 47(22):4144-4148. doi: 10.1002/(ISSN)1521-3773 [17] 梁方方, 周凌云, 李想, 樊静.乙二胺改性金属有机骨架材料MIL-101(Cr) 常压下吸附CO2[J].过程工程学报, 2015, 15(6):1069-1074. http://www.jproeng.com/CN/abstract/abstract747.shtmlLIANG Fang-fang, ZHOU Ling-yun, LI Xiang, FAN Jing. Adsorption of CO2 on ethylenediamine modified metal-organic framework material MIL-101(Cr) under atmospheric pressure[J]. Chin J Proc Eng, 2015, 15(6):1069-1074. http://www.jproeng.com/CN/abstract/abstract747.shtml [18] LIN Y, LIN H, WANG H, SUO Y, LI B, KONG C, CHEN L. Enhanced selective CO2 adsorption on polyamine/MIL-101(Cr) composites[J]. J Mater Chem A, 2014, 2(35):14658-14665. doi: 10.1039/C4TA01174K [19] HONG D Y, HWANG Y K, SERRE C, FÉREY G, CHANG J S. Porous chromium terephthalate MIL-101 with coordinatively unsaturated sites:Surface functionalization, encapsulation, sorption and catalysis[J]. Adv Funct Mater, 2009, 19(10):1537-1552. doi: 10.1002/adfm.v19:10 [20] 陈琳琳, 王霞, 郭庆杰.四乙烯五胺修饰介孔硅胶吸附CO2性能的研究[J].燃料化学学报, 2015, 43(1):108-115. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18563.shtmlCHEN Lin-lin, WANG Xia, GUO Qing-jie. Study on CO2 adsorption properties of tetraethylenepentamine modified mesoporous silica gel[J]. J Fuel Chem Technol, 2015, 43(1):108-115. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18563.shtml [21] 陈恒. 咪唑化合物辅助合成金属有机骨架MIL-101及其CO2吸附性能研究[D]. 大连: 大连理工大学, 2014.CHEN Heng. Synthesis and CO2 Adsorption Property of Metal-organic Framework MIL-101 by an Imidazole-assistant Route[D]. Dalian:Dalian University of Tecnology, 2014. [22] LIANG Z, MARSHALL M, NG C H, CHAFFEE A L. Comparison of conventional and HF-free-synthesized MIL-101 for CO2 adsorption separation and their water stabilities[J]. Energy Fuels, 2013, 27(12):43-59. https://www.researchgate.net/publication/263947425_Comparison_of_Conventional_and_HF-Free-Synthesized_MIL-101_for_CO2_Adsorption_Separation_and_Their_Water_Stabilities [23] 胡航标, 张涛, 崔征, 唐盛伟.氧化石墨烯-羧基碳纳米管-多乙烯多胺三维蜂窝状材料吸附CO2[J].化工进展, 2016, 35(11):3576-3584. http://mall.cnki.net/magazine/Article/HGJZ201611031.htmHU Hang-biao, ZHANG Tao, CUI Zheng, TANG Sheng-wei. Preparation of three-dimensional honeycomb-like material of Graphene oxide-carboxylated carbon nanotube-polyethylenepolyamine to adsorb CO2[J]. Chem Ind Eng Prog, 2016, 35(11):3576-3584. http://mall.cnki.net/magazine/Article/HGJZ201611031.htm -





 下载:
下载: