Preparation of C9H10O2-0.5ZnCl2/Al2O3 and its oxidative desulfurization performance
-
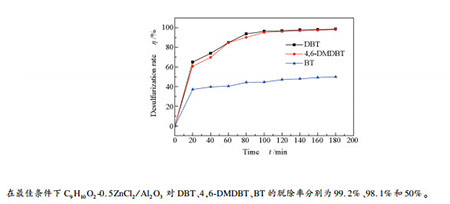
摘要: 通过将C9H10O2-0.5ZnCl2双酸型低共熔溶剂固载到Al2O3上制备了C9H10O2-0.5ZnCl2/Al2O3催化剂。该催化剂采用XRD、FT-IR、SEM、EDS、N2吸附-脱附技术进行了分析。以C9H10O2-0.5ZnCl2/Al2O3为催化剂,过氧化氢为氧化剂研究模拟油中芳香族硫化物的脱除性能。考察反应参数如温度、催化剂加入量、O/S物质的量比、硫化物类型等对催化剂脱硫活性的影响。实验结果表明,在模拟油为5 mL、催化剂量为0.2 g、O/S比为8、反应温度为60 ℃、反应时间为180 min的条件下,模拟油中二苯并噻吩(DBT)脱硫率为99.2%。此外,在模拟油氧化脱硫中催化剂循环使用六次,其氧化脱硫活性略有降低。研究了C9H10O2-0.5ZnCl2/Al2O3催化氧化脱硫的反应机理。Abstract: A C9H10O2-0.5ZnCl2/Al2O3 catalyst was successfully prepared by immobilizing phenylpropionic acid-zinc chloride(C9H10O2-0.5ZnCl2) double acid deep eutectic solvent on Al2O3, and analyzed by XRD, FT-IR, SEM, EDS and N2 adsorption-desorption. The removal activity for aromatic sulfides in model oil using C9H10O2-0.5ZnCl2/Al2O3 as catalysis and H2O2 as oxidant and the effect of some reaction parameters such as temperature, catalyst dosage, O/S molar ratio and different sulfide types on the desulfurization activity of catalyst were investigated. The experimental results show that with the model oil of 5 mL, the catalyst dosage of 0.2 g and the O/S molar ratio of 8, at temperature of 60℃and reaction time of 180 min, the removal rate of DBT can reach to 99.2%. In addition, the catalyst can be recycled up to 6 times with a little decrease in catalytic activity for the ODS process. The catalysis-oxidation desulfurization mechanism of C9H10O2-0.5ZnCl2/Al2O3 was also explored.
-
Key words:
- zinc chloride /
- alumina /
- oxidation desulfurization /
- deep eutectic solvents
-
表 1 样品的表面结构表征
Table 1 Surface structural characteristics of samples
Sample Surface A/(m2·g-1) Pore volume v/(cm3·g-1) Pore size d/nm Al2O3 346.5 1.164 0.5086 C9H10O2-0.5ZnCl2/Al2O3 171.4 0.5684 0.9976 表 2 不同脱硫体系脱硫活性对比
Table 2 Comparison of desulfurization performance of different desulfurization systems
Desulfurization systems Removal rate of DBT η/% H2O2/Al2O3[a] 20 H2O2/C9H10O2-0.5ZnCl2[b] 38 H2O2/C9H10O2-0.5ZnCl2/Al2O3[c] 99.2 reaction condition: a: 5 mL model oil, 60 ℃, O/S= 8, 0.133 g Al2O3, t=180 min; b: 5 mL model oil, 60 ℃, O/S= 8, 0.067 g C9H10O2-0.5ZnCl2, t=180 min; c: 5 mL model oil, 60 ℃, O/S= 8, 0.2 g C9H10O2-0.5ZnCl2/Al2O3, t=180 min 表 3 C9H10O2-0.5ZnCl2/Al2O3的重复使用性能
Table 3 Recycling performance of C9H10O2-0.5ZnCl2 /Al2O3
Recycle times 1 2 3 4 5 6 Sulfur removal η/% 99.2 96.2 95.4 92.3 88.3 85.1 -
[1] BOKARE A D, CHOI W. Bicarbonate-induced activation of H2O2 for metal-free oxidative desulfurization[J]. J Hazard Mater, 2016, 304:313-319. doi: 10.1016/j.jhazmat.2015.10.063 [2] ANDEVARY H H, AKBARI A, OMIDKHAH M. High efficient and selective oxidative desulfurization of diesel fuel using dual-function[Omim]FeCl4 as catalyst/extractant[J]. Fuel Process Technol, 2019, 185:8-17. doi: 10.1016/j.fuproc.2018.11.014 [3] 林燕, 王芳, 张志庆, 杨洁, 魏颖.离子液体绿色脱硫机理及应用进展[J].化工进展, 2013, 32(3):549-557. http://d.old.wanfangdata.com.cn/Periodical/hgjz201303010LIN Yan, WANG Fang, ZHANG Zhi-qing, YANG Jie, WEI Ying. Mechanism and application of ionic liquids in environmental friendly oil desulphurization[J]. Chem Ind Eng Prog, 2013, 32(3):549-557. http://d.old.wanfangdata.com.cn/Periodical/hgjz201303010 [4] JI H Y, SUN J, WU P W, WU Y C, HE J, CHAO Y H, ZHU W S, LI H M. Silicotungstic acid immobilized on lamellar hexagonal boron nitride for oxidative desulfurization of fuel components[J]. Fuel, 2018, 213:12-21. doi: 10.1016/j.fuel.2017.08.076 [5] 李文秀, 催安磊, 范俊刚, 孙向乐, 张志刚.载铜球形活性炭的制备及其吸附脱硫性能的研究[J].燃料化学学报, 2013, 41(5):613-618. doi: 10.3969/j.issn.0253-2409.2013.05.013LI Wen-xiu, CUI An-lei, FAN Jun-gang, SUN Xiang-le, ZHANG Zhi-gang. Synthesis of spherical activated carbon supported copper catalyst and its performance for adsorptive desulfurization[J]. J Fuel Chem Technol, 2013, 41(5):613-618. doi: 10.3969/j.issn.0253-2409.2013.05.013 [6] TIAN Y, WANG G, LONG J, CUI J W, JIN W, ZENG D L. Ultra-deep oxidative desulfurization of fuel with H2O2 catalyzed by phosphomolybdic acid supported on silica[J]. Chin J Catal, 2016, 37(12):2098-2105. doi: 10.1016/S1872-2067(16)62558-5 [7] LI S W, YANG Z, GAO R M, ZHANG G, ZHAO J S. Direct synthesis of mesoporous SRL-POM@MOF-199@MCM-41 and its highly catalytic performance for the oxidesulfurization of DBT[J]. Appl Catal B:Environ, 2018, 221:574-583. doi: 10.1016/j.apcatb.2017.09.044 [8] ZHU W S, WANG C, LI H M, WU P W, XUN S H, JIANG W, CHEN Z G, ZHAO Z, LI H M. One-pot extraction combined with metal-free photochemical aerobic oxidative desulfurization in deep eutectic solvent[J]. Green Chem, 2015, 17:2464-2472. doi: 10.1039/C4GC02425G [9] LI S W, LI J R, GAO Y, LIANG L L, ZHANG R L, ZHAO J S. Metal modified heteropolyacid incorporated into porous materials for a highly oxidative desulfurization of DBT under molecular oxygen[J]. Fuel, 2017, 197:551-561. doi: 10.1016/j.fuel.2017.02.064 [10] STANISLAUS A, MARAFI A, RANA M S. Recent advances in the science and technology of ultra low sulfur diesel (ULSD) production[J]. Catal Today, 2010, 153(1/2):1-68. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=c3a5d46f7b195464cfb15c24c522231a [11] KANG L, LIU H G, HE H J, YANG C P. Oxidative desulfurization of dibenzothiophene using molybdenum catalyst supported on Ti-pillared montmorillonite and separation of sulfones by filtration[J]. Fuel, 2018, 234:1229-1237. doi: 10.1016/j.fuel.2018.07.148 [12] YANG C P, ZHAO K, CHENG Y, ZENG G M, ZHANG M M, SHAO J J, LU L. Catalytic oxidative desulfurization of BT and DBT from n-octane using cyclohexanone peroxide and catalyst of molybdenum supported on 4A molecular sieve[J]. Sep Purif Technol, 2016, 163:153-161. doi: 10.1016/j.seppur.2016.02.050 [13] WEI S N, HE H J, CHENG Y, YANG C P, ZENG G M, KANG L, QIAN H, ZHU C Y. Preparation, characterization, and catalytic performances of cobalt catalysts supported on KIT-6 silicas in oxidative desulfurization of dibenzothiophene[J]. Fuel, 2017, 200:11-21. doi: 10.1016/j.fuel.2017.03.052 [14] CHOI A E S, ROCES S, DUGOS N, WAN M W. Oxidation by H2O2 of bezothiophene and dibenzothiophene over different polyoxometalate catalysts in the frame of ultrasound and mixing assisted oxidative desulfurization[J]. Fuel, 2016, 180:127-136. doi: 10.1016/j.fuel.2016.04.014 [15] ABBOTT A P, ALAYSUY O, ANTUNES A P M, DOUGLAS A C, GUTHRIE-STRACHAN J, WISE W R. Processing of leather using deep eutectic solvents[J]. ACS Sustainable Chem Eng, 2015, 3:1241-1247. doi: 10.1021/acssuschemeng.5b00226 [16] ABBOTT A P, BOOTHBY D, CAPPER G, DAVIES D L, RASHEED R K. Deep eutectic solvents formed between choline chloride and carboxylic acids:versatile alternatives to ionic liquids[J]. J Am Chem Soc, 2004, 126(29):9142-9147. doi: 10.1021/ja048266j [17] ZHANG Q, VIGIER K D O, ROYER S, JÉRÔME F. Deep eutectic solvents:Syntheses, properties and applications[J]. Chem Soc Rev, 2012, 41(21):7108-7146. doi: 10.1039/c2cs35178a [18] GUAJARDO N, CARLESI C, ARACENA Á. Toluene oxidation by hydrogen peroxide in deep eutectic solvents[J]. ChemCatChem, 2015, 7(16):2451-2454. doi: 10.1002/cctc.201500604 [19] DAI Y, WITKAMP G J, VERPOORTE R, CHOI Y H. Natural deep eutectic solvents as a new extraction media for phenolic metabolites in Carthamus tinctorius L[J]. Anal Chem, 2013, 85(13):6272-6278. doi: 10.1021/ac400432p [20] NKUKU C A, LESUER R J. Electrochemistry in deep eutectic solvents[J]. J Phys Chem B, 2007, 111(46):13271-13277. doi: 10.1021/jp075794j [21] GARCIA G, ATILHAN M, APARICIO S. Interfacial properties of deep eutectic solvents regarding to CO2 capture[J]. J Phys Chem C, 2015, 119(37):21413-21425. doi: 10.1021/acs.jpcc.5b04585 [22] LIU P, HAO J W, MO L P, ZHANG Z H. Recent advances in the application of deep eutectic solvents as sustainable media as well as catalysts in organic reactions[J]. RSC Adv, 2015, 5(60):48675-48704. doi: 10.1039/C5RA05746A [23] 侯良培, 赵荣祥, 李秀萍, 石薇薇.甲基咪唑盐酸盐/草酸型低共熔溶剂的制备及其在模拟油中氧化脱硫中的应用[J].化工学报, 2016, 67(9):3972-3980. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hgxb201609056HUO Liang-pei, ZHAO Rong-xiang, LI Xiu-ping, SHI Wei-wei. Preparation of methylimidazole hydrochloride/oxalic acid type deep eutectic solvent and its application in oxidative desulfurization of model oil[J]. J Chem Ind Eng, 2016, 67(9):3972-3980. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hgxb201609056 [24] SHAH D, GAPEYENKO D, URAKPAYEV A, TORKMAHALLEH M. Molecular dynamics simulations on extractive desulfurization of fuels by tetrabutylammonium chloride based Deep Eutectic Solvents[J]. J Mol Liq, 2019, 274:254-260. doi: 10.1016/j.molliq.2018.10.131 [25] HAO L, SU T, HAO D, DENG C L, REN W Z, LÜ H Y. Oxidative desulfurization of diesel fuel with caprolactam-based acidic deep eutectic solvents:Tailoring the reactivity of DESs by adjusting the composition[J]. Chin J Catal, 2018, 39(9):1552-1559. doi: 10.1016/S1872-2067(18)63091-8 [26] MAO C F, ZHAO R X, LI X P. Phenylpropanoic acid-based DESs as efficient extractants and catalysts for the removal of sulfur compounds from oil[J]. Fuel, 2017, 189:400-407. doi: 10.1016/j.fuel.2016.10.113 [27] MAO C F, ZHAO R X, LI X P. Propionic acid-based deep eutectic solvents:Synthesis and ultra-deep oxidative desulfurization activity[J]. RSC Adv, 2017, 7(67):42590-42596. doi: 10.1039/C7RA05687G [28] LIU Z M, WANG J W, KANG M Q, YIN N, WANG X H, TAN Y S, ZHU Y L. Synthesis of glycerol carbonate by transesterification of glycerol and dimethyl carbonate over KF/γ-Al2O3 catalyst[J]. J Braz Chem Soc, 2014, 25(1):152-160. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=jxhg201210020 [29] AKBARI A, OMIDKHAH M, DARIAN J T. Investigation of process variables and intensification effects of ultrasound applied in oxidative desulfurization of model diesel over MoO3/Al2O3 catalyst[J]. Ultrason Sonochem, 2014, 21(2):692-705. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=8630a820a4b86ada2120488e9f289207 [30] ZHAO D, WANG J, ZHOU E. Oxidative desulfurization of diesel fuel using a Brønsted acid room temperature ionic liquid in the presence of H2O2[J]. Green Chem, 2007, 9(11):1219-1222. doi: 10.1039/b706574d [31] AL-SHAHRANI F, XIAO T, LLEWELLYN S A, BARRI S, JIANG Z, SHI H H, MARTINIE G, L H GREEN M. Desulfurization of diesel via the H2O2 oxidation of aromatic sulfides to sulfones using a tungstate catalyst[J]. Appl Catal B:Environ, 2007, 73(3/4):311-316. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=0a99ccabcb293c5620972f14e82e97b5 [32] JIANG X, LI H M, ZHU W, HE L N, SHU H M, LU J D. Deep desulfurization of fuels catalyzed by surfactant-type decatungstates using H2O2 as oxidant[J]. Fuel, 2009, 88(3):431-436. doi: 10.1016/j.fuel.2008.11.010 [33] XIE D, HE Q H, SU Y Y, WANF T W, XU R F, HU B J. Oxidative desulfurization of dibenzothiophene catalyzed by peroxotungstate on functionalized MCM-41 materials using hydrogen peroxide as oxidant[J]. Chin J Catal, 2015, 36(8):1205-1213. doi: 10.1016/S1872-2067(15)60897-X [34] DAI B L, WU P W, ZHU W S, CHAO YH, SUN J, XIONG J, JIANG W, LI H M. Heterogenization of homogenous oxidative desulfurization reaction on graphene-like boron nitride with a peroxomolybdate ionic liquid[J]. RSC Adv, 2016, 6(1):140-147. doi: 10.1039/C5RA23272D [35] 张薇, 丁永萍, 宫摇敬, 宋溪明.羧基功能化离子液体催化二苯并噻吩氧化脱硫[J].燃料化学学报, 2012, 40(5):628-632. http://www.cnki.com.cn/Article/CJFDTotal-RLHX201205020.htmZHANG Wei, DING Yong-ping, GONG Yao-jing, SONG Xi-ming. Oxidative desulfurization of dibenzothiophene catalyzed by carboxyl-functionalized ionic liquid[cmmim]BF4[J]. J Fuel Chem Technol, 2012, 40(5):628-632. http://www.cnki.com.cn/Article/CJFDTotal-RLHX201205020.htm [36] HAO L W, WANG M R, SHAN W J, DENG C L, REN W Z, SHI Z Z, LÜ H Y. L-proline-based deep eutectic solvents (DESs) for deep catalytic oxidative desulfurization (ODS) of diesel[J]. J Hazard Mater, 2017, 339:216-222. doi: 10.1016/j.jhazmat.2017.06.050 [37] 项小燕, 林金清.离子液体萃取燃料油脱硫技术的研究进展[J].化工进展, 2007, 26(12):1681-1685. doi: 10.3321/j.issn:1000-6613.2007.12.003XIANG Xiao-yan, LIN Jin-qin. Progress of extractive desulfurization technology with ionic liquids[J]. Chem Ind Eng Prog, 2007, 26(12):1681-1685. doi: 10.3321/j.issn:1000-6613.2007.12.003 -





 下载:
下载: