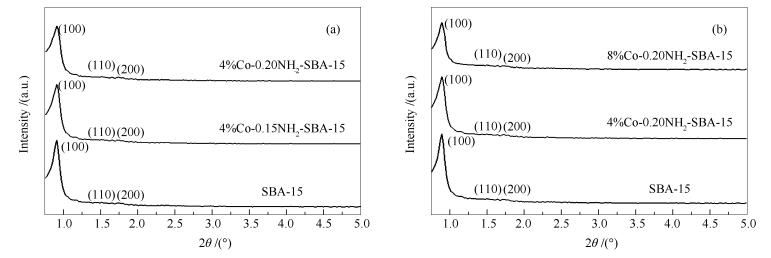
-
摘要: 采用水热合成法制备介孔分子筛SBA-15,用(CH3COO)2Co对其进行超声浸渍改性,并用硅烷偶联剂APTS将氨基引入SBA-15分子筛中,制备出Co-NH2-SBA-15吸附剂,考察了常温条件下H2S的吸附性能。通过SEM、XRD、FT-IR、BET、XPS等表征手段对吸附剂进行表征。结果表明,氨基与金属同时负载在分子筛表面,氨基与硅物质的量比为0.20,Co负载质量分数为8%的SBA-15吸附效果最好,当原料气H2S体积分数为227 μL/L,温度25 ℃,气体流量75 mL/min时,穿透硫容和饱和硫容达0.151和0.190 mmol/g,且吸附剂可再生利用。SBA-15表面嫁接氨基并浸渍金属改性的手段不仅提高了吸附容量,同时提高了分子筛的稳定性。Abstract: The mesoporous molecular sieve SBA-15 was prepared by hydrothermal synthesis, modified with the ultrasonic immersion by (CH3COO)2Co and with the introduction of the amino group into it with APTS as silane coupling agent, and characterized by SEM, XRD, FT-IR, BET and XPS. The prepared Co-NH2-SBA-15 was used for adsorption of H2S at normal temperature. The results show that both amino group and metal have been introduced into the molecular sieve. When the molar ratio of amino group to silicon is 0.20 and the Co loading is 8%, the adsorption capacity of SBA-15 is the highest. Under the conditions of the volume fraction of hydrogen sulfide of 227 μL/L, the temperature of 25 ℃ and the gas flow rate of 75 mL/min, the capacities of breakthrough sulfur and saturated sulfur come to 0.151 and 0.190 mmol/g, respectively. Moreover, the adsorbent can be regenerated for recycle. Under the assistance of molecular sieve surface being grafted by amino group and impregnated by metal, the adsorption capacity can be improved and the stability of molecular sieve can be enhanced.
-
Key words:
- SBA-15 /
- modified /
- APTS /
- H2S adsorption
-
表 1 SBA-15及改性后样品的BET表征
Table 1 BET data of SBA-15 and its modified samples
Sample Surface area A/(m2·g-1) Pore volume v/(cm3·g-1) Pore diameter d/nm SBA-15 796.6 0.9579 7.6 4%Co-0.20NH2-SBA-15 551.4 0.8377 6.4 8%Co-0.20NH2-SBA-15 292.4 0.3488 5.2 表 2 不同负载量Co-NH2-SBA-15的吸附性能
Table 2 H2S adsorption performance of Co-NH2-SBA-15 with different Co loadings
Adsorbent Breakthrough time t/min Saturation time t/min Cap(BT)/(mmol·g-1) Cap(S)/(mmol·g-1) SBA-15 2.4 6.6 0.002 0.007 0.15NH2-SBA-15 36.2 46.2 0.037 0.047 4%Co-0.20NH2-SBA-15 49.8 62.8 0.042 0.064 6%Co-0.15NH2-SBA-15 70.2 88.2 0.072 0.090 6%Co-0.20NH2-SBA-15 84.6 98.6 0.086 0.101 8%Co-0.25NH2-SBA-15 106.2 150.2 0.108 0.153 8%Co-0.20NH2-SBA-15 148.1 186.1 0.151 0.190 10%Co-0.20NH2-SBA-15 134.8 199.8 0.137 0.204 -
[1] 王传亮, 杨青扬, 周善柯, 潘摇鑫, 詹冬武, 王旭珍.煤基氮掺杂介孔炭的制备及其室温催化氧化脱除H2S性能[J].燃料化学学报, 2018, 46(1):111-118. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract19158.shtmlWANG Chuan-liang, YANG Qing-yang, ZHOU Shan-ke, PAN Yao-xin, ZHAN Dong-wu, WANG Xu-zhen. Preparation of coal derived nitrogen-doped mesoporous carbon for the catalytic oxidation of H2S at room temperature[J]. J Fuel Chem Technol, 2018, 46(1):111-118. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract19158.shtml [2] 李自力, 程远鹏, 毕海胜, 董有智.油气田CO2/H2S共存腐蚀与缓蚀技术研究进展[J].化工学报, 2014, 65(2):406-414. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hgxb201402006LI Zi-li, CHENG Yuan-peng, BI Hai-sheng, DONG You-zhi. Research progress of CO2/H2S corrosion and inhibitor techniques in oil and gas fields[J]. J Chem Ind Eng, 2014, 65(2):406-414. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hgxb201402006 [3] MINIER-MATAR J, JANSON A, HUSSAIN A, ADHAM S. Application of membrane contactors to remove hydrogen sulfide from sour water[J]. J Membranc Sci, 2017, 541(1):378-385. [4] SU H J, WANG S D, NIU H N, PAN L W, WANG A J, HU Y K. Mass transfer characteristics of H2S absorbtion from gaseous mixture into methyldiethanolamine solution in a T-junction microchannel[J]. Sep Purif Technol, 2010, 72(3):326-334. doi: 10.1016/j.seppur.2010.02.024 [5] KUZNETSOVA T F, IVANETS A I, KUL'BITSKAYA L V, BUDEIKO N L, SAVKA Y D. Synthesis and characterization of homogeneously mesoporous magnesium silicates with prospects of application in catalysis and adsorption[J]. Prot Met Phys Chem, 2017, 53(4):651-656. doi: 10.1134/S2070205117040116 [6] CHMIELARZ L, KUSTROWSKI P, DROZDEK M, DZIEMBAJ R, COOL P, VANSANT E F. Selective catalytic oxidation of ammonia into nitrogen over PCH modified with copper and iron species[J]. Catal Today, 2008, 114(2/3):319-325. [7] 赵东元, 万颖, 周武纵.有序介孔分子筛材料[M].北京:高等教育出版社, 2013:326-334.ZHAO Dong-yuan, WAN Ying, ZHOU Wu-zong. Ordered Mesoporous Molecular Sieve Materials[M]. Beijing:Higher Education Press, 2013:326-334. [8] LI Y T, KEEFE A J, GIARMARCO M, BRAULT N D, JIANG S Y. Simple and robust approach for passivating and functionalizing surfaces for use in complex media[J]. Langmuir, 2012, 28(25):9707-9713. doi: 10.1021/la301691d [9] YANAGISAWA T, SHIMIZU T, KURODA K. The preparation of alkyltriinethylaininonium-kaneinite complexes and their conversion to microporous materials[J]. Bull Chem Soa Jpn, 1990, 63(4):988-992. doi: 10.1246/bcsj.63.988 [10] ZHAO D Y, HUO Q S, FENG J L, B F C, G D S. Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures[J]. J Am Chem Soc, 1998, 136(29):6024-6036. [11] BELMABKHOUT Y, HEYMANS N, WEIRELD G D, SAYARI A. Simultaneous adsorption of H2S and CO2 on triamine-grafted pore-expanded mesoporous MCM-41 silica[J]. Energy Fuels, 2011, 25(3):1310-1315. doi: 10.1021/ef1015704 [12] 陈颖, 乔腾飞, 姬生伦, 苗双, 张宏宇.混合胺改性SBA-15的制备及其吸附脱硫特性[J].石油学报(石油加工), 2016, 32(5):883-890. http://www.oalib.com/paper/4213696CHEN Ying, QIAO Teng-fei, JI Sheng-lun, MIAO Shuang, ZHANG Hong-yu. Adsorption of H2S by mixed-amine functionalized SBA-15[J]. Acta Pet Sin (Pet Process Sect), 2016, 32(5):883-890. http://www.oalib.com/paper/4213696 [13] 李勇, 李磊, 闻霞, 王峰, 赵宁.二次嫁接法制备氨基修饰的硅基二氧化碳吸附剂[J].燃料化学学报, 2013, 41(9):1122-1128. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18261.shtmlLI Yong, LI Lei, WEN Xia, WANG Feng, ZHAO Ning. Synthesis of amine modified silica for the capture of carbon dioxide by a twice grafting method[J]. J Fuel Chem Technol, 2013, 41(9):1122-1128. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18261.shtml [14] MATHEW A, PARAMBADATH S, SU Y K, HA H M, HA C S. Diffusion mediated selective adsorption of Zn2+ from artificial seawater by MCM-41[J]. Microporous Mesoporous Mater, 2016, 229:124-133. doi: 10.1016/j.micromeso.2016.04.028 [15] SHAHBAZI A, YOUNESI H, BADIEI A. Functionalized SBA-15 mesoporous silica by melamine-based dendrimer amines for adsorptive characteristics of Pb(Ⅱ), Cu(Ⅱ) and Cd(Ⅱ) heavy metal ions in batch and fixed bed column[J]. Chem Eng J, 2011, 168(2):505-518. doi: 10.1016/j.cej.2010.11.053 [16] 纪桂杰, 张耀兵, 付宁宁. Mn/Al-SBA-15的制备及吸附脱硫性能[J].燃料化学学报, 2015, 43(4):449-455. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18609.shtmlJI Gui-jie, ZHANG Yao-bing, FU Ning-ning. Study on the preparation of Mn/Al-SBA-15 and its adsorpt ion desulfurization performance[J]. J Fuel Chem Technol, 2015, 43(4):449-455. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18609.shtml [17] WANG Y, NOGUCHI M, TAKAHASHI Y. Synthesis of SBA-15 with different pore sizes and the utilization as supports of high loading of cobalt catalysis[J]. Catal Today, 2001, 68(1/3):3-9. [18] KHODAKOV A Y, BECHARA R, GRIBOVAL-CONSTANT A. Fischer-Tropsch synthesis over silica supported cobalt catalysts:Mesoporous structure versus cobalt surface density[J]. Appl Catal A:Gen, 2003, 254(2):273-288. doi: 10.1016/S0926-860X(03)00489-7 [19] CHEN S Y, HUANG C Y, YOKOI T. Synthesis and catalytic activity of amino-functionalized SBA-15 materials with controllable channel lengths and amino loadings[J]. J Mater Chem A, 2012, 22(5):2233-2243. doi: 10.1039/C2JM14393C [20] CANO L A, CAGNOLI M V, BENGOA J F, GARCIA-FIERRO J L, MARCHETTI S G. Synthesis and characterization of SBA-15 modified with alkali metals[J]. J Porous Mater, 2016:1-8. [21] 魏建文, 韦真周, 廖雷, 赵淞盛, 王敦球.氨基修饰介孔分子筛SBA-15对水中Pb2+吸附性能[J].环境工程学报, 2014, 8(5):825-1830.WEI Jian-wen, WEI Zhen-zhou, LIAO Lei, ZHAO Song-sheng, WANG Dun-qiu. Aqueous Pb (Ⅱ) removal by adsorption on amine-functionalized mesoporous silica SBA-15[J]. Chin J Environ Eng, 2014, 8(5):1825-1830. [22] 魏诠, 应春钟.载钴分子筛的XPS研究[J].高等学校化学学报, 1991, 2(1):80-83. http://www.cqvip.com/QK/90335X/199101/483054.htmlWEI Quan, YING Chun-zhong. XPS study of cobalt molecular sieve[J]. Chem Res Chin Univ, 1991, 2(1):80-83. http://www.cqvip.com/QK/90335X/199101/483054.html [23] 周玮, 房克功, 陈建刚, 孙予罕.水对钴基Fischer-Tropsch合成的影响[J].化学进展, 2006, 18(1):45-50. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hxjz200601007ZHOU Wei, FANG Ke-gong, CHEN Jian-gang, SUN Yu-han. Effects of water on the synthesis of cobalt-based Fischer-Tropsch[J]. Chem Eng Prog, 2006, 18(1):45-50. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hxjz200601007 -





 下载:
下载: