Synthesis of RUB-13 zeolite and its catalytic performance in the conversion of methanol to olefins
-
摘要: 采用水热法合成RUB-13分子筛,探讨了有机模板剂(OSDA)、硅源、晶化温度和水硅比等制备条件对RUB-13分子筛晶体结构的影响,考察了RUB-13分子筛在甲醇制烯烃(MTO)反应中的催化性能。结果表明,采用1,2,2,6,6-五甲基哌啶(PMP)为有机模板剂、白炭黑为硅源,在晶化温度为170℃的条件下,选择H2O/Si比为100和80时可分别合成出高纯度的低硅铝比(Si/Al=100)和高硅铝比(Si/Al=200)的RUB-13分子筛晶体,且晶粒呈棒状形貌。H-Al-B-RUB-13(Si/Al=200)分子筛用于催化甲醇制烯烃反应时,在400℃下表现出高的低碳烯烃选择性(C2-5=选择性达97.8%,丙烯选择性为54.5%),优于传统的H-SAPO-34和H-ZSM-5分子筛催化剂。Abstract: RUB-13 zeolites were synthesized by hydrothermal method. The effect of organic structure-directing agent (OSDA), silica source, crystallization temperature and Si/H2O ratio on the crystal structure was investigated and the catalytic performance of H-RUB-13 in the conversion of methanol to olefins (MTO) was evaluated. The results indicate that with 1, 2, 2, 6, 6-pentamethylpiperidine (PMP) as OSDA and fumed silica as silica source, pure and highly crystalline RUB-13 zeolites with a Si/Al ratio of 100 or 200 can be successfully synthesized by crystallization at 170 ℃, with a H2O/Si ratio of 100 or 80 in the synthesis gel, respectively. The crystals of the synthesized RUB-13 zeolites display a thin rod-like morphology. As a catalyst in MTO, the high-silica H-Al-B-RUB-13 (Si/Al=200) zeolite exhibits unprecedentedly high selectivity to light olefins at 400 ℃ (54.5% to propene and 97.8% to C2-5=), much better than the traditional H-SAPO-34 and H-ZSM-5 zeolites under similar conditions.
-
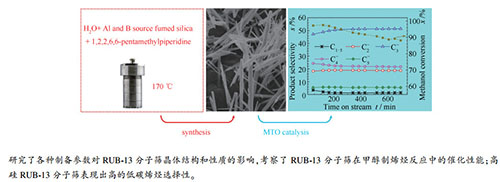
图 6 不同硅铝比的H-Al-B-RUB-13分子筛催化剂上MTO反应过程中的甲醇转化率和产物选择性
Figure 6 Methanol conversion and product selectivity with time on stream for MTO over the H-Al-B-RUB-13 zeolites with different Si/Al ratios
(a): H-Al-B-RUB-13(Si/Al=100); (b): H-Al-B-RUB-13(Si/Al=200) the reactions were carried out at 400 ℃, with a methanol weight space velocity of 1.0 h-1
表 1 不同硅铝比H-Al-B-RUB-13分子筛的组成结构和酸性
Table 1 Constitution and acidity of the H-Al-B-RUB-13 zeolites with different Si/Al ratios synthesized under optimized conditions
Zeolite Si/Al ratio Si/B ratio Acidity by NH3-TPD/ (μmol·g-1) gel bulk gel bulk weak strong H-Al-B-RUB-13(Si/Al=100) 100 70 4 20 71 89 H-Al-B-RUB-13(Si/Al=200) 200 171 4 18 34 56 note:the elemental compositions of the synthesis gel and the bulk H-Al-B-RUB-13 zeolite were determined by ICP-OES, the amounts of weak and strong acid sites were determined as the quantities of ammonia desorbed at 120-250 and 250-550 ℃ during the NH3-TPD, respectively -
[1] CUNDY C S, COX P A. The hydrothermal synthesis of zeolites:History and development from the earliest days to the present time[J]. Chem Rev, 2003, 103(3):663-702. doi: 10.1021/cr020060i [2] CORMA A. State of the art and future challenges of zeolites as catalysts[J]. J Catal, 2003, 216(1/2):298-312. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=c3a0d63140c3dc2c29aa936cf6b1e489 [3] 慕旭宏, 王殿中, 王永睿, 林民, 程时标, 舒兴田.纳米分子筛在炼油和石油化工中的应用[J].催化学报, 2013, 34(1):69-79. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=cuihuaxb201301007MU Xu-hong, WANG Dian-zhong, WANG Yong-rui, LIN Min, CHENG Shi-biao, SHU Xing-tian. Nanosized molecular sieves as petroleum refining and petrochemical catalysts[J]. Chin J Catal, 2013, 34(1):69-79. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=cuihuaxb201301007 [4] 程志林, 晁自胜, 万惠霖.气体分离分子筛膜[J].化学进展, 2004, 16(1):61-67. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hxjz200401010CHENG Zhi-lin, CHAO Zi-sheng, WAN Hui-lin. Progress in the research of zeolite membrane on gas separation[J]. Prog Chem, 2004, 16(1):61-67. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hxjz200401010 [5] ZHU Q, KONDO J N, OHNUMA R, KUBOTA Y, YAMAGUCHI M, TATSUMI T. The study of methanol-to-olefin over proton type aluminosilicate CHA zeolites[J]. Microporous Mesoporous Mater, 2008, 112(1/3):153-161. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=cc7d73c543f52e0afd687143c7a618d7 [6] ZHU Q, HINODE M, YOKOI T, KONDO J N, KONDO Y, TATSUMI T. Methanol-to-olefin over gallosilicate analogues of chabazite zeolite[J]. Microporous Mesoporous Mater, 2008, 116(1/3):253-257. http://www.sciencedirect.com/science/article/pii/S138718110800187X [7] LIANG J, LI H, ZHAO S, GUO W, WANG R, YING M. Characteristics and performance of SAPO-34 catalyst for methanol-to-olefin conversion[J]. Appl Catal, 1990, 64:31-40. doi: 10.1016/S0166-9834(00)81551-1 [8] ZHU Q, KONDO J N, TATSUMI T, INAGAKI S, OHNUMA R, KUBOTA Y, SHIMODAIRA Y, DOMEN K. A comparative study of methanol to olefin over CHA and MTF zeolites[J]. J Phys Chem C, 2007, 111(14):5409-5415. doi: 10.1021/jp063172c [9] 唐君琴, 叶丽萍, 应卫勇, 房鼎业.硅铝比对SAPO-34催化剂在甲醇制烯烃反应中催化性能的影响[J].石油化工, 2010, 39(1):22-27. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=syhg201001005TANG Jun-qin, YE Li-ping, YING Wei-yong, FANG Ding-ye. Performances of SAPO-34 catalysts with different ratios of Si to Al in methanol-to-olefins[J]. Petrochem Technol, 2010, 39(1):22-27. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=syhg201001005 [10] PARK J W, LEE J Y, KIM K S, HONG S B, SEO G. Effects of cage shape and size of 8-membered ring molecular sieves on their deactivation in methanol-to-olefin (MTO) reactions[J]. Appl Catal A:Gen, 2008, 339(1):36-44. doi: 10.1016/j.apcata.2008.01.005 [11] IVANOVA S, LEBRUN C, VANHAECKE E, PHAM-HUU C, LOUIS B. Influence of the zeolite synthesis route on its catalytic properties in the methanol to olefin reaction[J]. J Catal, 2009, 265(1):1-7. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=5719ec11f11a92d00cea5543a3d3ff31 [12] BLEKEN F L, CHAVAN S, OLSBYE U, BOLTZ M, OCAMPO F, LOUIS B. Conversion of methanol into light olefins over ZSM-5 zeolite:Strategy to enhance propene selectivity[J]. Appl Catal A:Gen, 2012, 447:178-185. http://www.sciencedirect.com/science/article/pii/S0926860X12006187 [13] YOKOI T, YOSHIOKA M, IMAI H, TATSUMI T. Diversification of RTH-type zeolite and its catalytic application[J]. Angew Chem Int Ed, 2009, 48(52):9884-9887. doi: 10.1002/anie.200905214 [14] YOSHIOKA M, YOKOI T, LIU M, IMAI H, INAGAKI S, TATSUMI T. Preparation of RTH-type zeolites with the amount and/or kind of organic structure-directing agents (OSDA):Are OSDAs indispensable for the crystallization?[J]. Microporous Mesoporous Mater, 2012, 153:70-78. doi: 10.1016/j.micromeso.2011.12.024 [15] LIU M, YOKOI T, YOSHIOKA M, IMAI H, KONDO J N, TATSUMI T. Differences in Al distribution and acidic properties between RTH-type zeolites synthesized with OSDAs and without OSDAs[J]. Phys Chem Chem Phys, 2014, 16(9):4155-4164. doi: 10.1039/c3cp54297a [16] ZHANG L, WANG S, SHI D, QIN Z, WANG P, WANG G, LI J, DONG M, FAN W, WANG J. Methanol to olefins over H-RUB-13 zeolite:Regulation of framework aluminum siting and acid density and their relationship to the catalytic performance[J]. Catal Sci Technol, 2020, 10(6):1835-1847. doi: 10.1039/C9CY02419K [17] 贾艳萍, 马姣, 张兰河, 董长青, 王孝强.多孔二氧化硅材料的应用进展[J].硅酸盐通报, 2014, 33(12):3206-3212. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=gsytb201412026JIA Yan-ping, MA Jiao, ZHANG Lan-he, DONG Chang-qing, WANG Xiao-qiang. Application progress on porous silica material[J]. Bull. Chin Ceram Soc, 2014, 33(12):3206-3212. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=gsytb201412026 [18] LANDRY C C, TOLBERT S H, GALLIS K W, MONNIER A, STUCKY G D, NORBY P, HANSON J C. Phase transformations in mesostructured silica/surfactant composites. Mechanisms for change and applications to materials synthesis[J]. Chem Mater, 2001, 13(5):1600-1608. doi: 10.1021/cm000373z [19] VORTMANN S, MARLER B, GIES H, DANIELS P. Synthesis and crystal structure of the new borosilicate zeolite RUB-13[J]. Microporous Mesoporous Mater, 1995, 4(2/3):111-121. http://www.sciencedirect.com/science/article/pii/092765139400090I -





 下载:
下载: