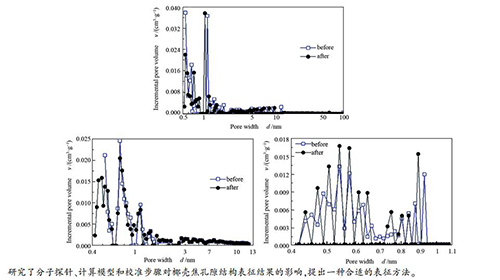
-
摘要: 为了更深入地了解微孔生物质焦的孔隙结构特征,在水蒸气气氛下制备椰壳焦(CSCs),并且采用了不同分子探针、计算模型和校准步骤对其进行表征。结果表明,椰壳焦有较高的碳含量和比较丰富的孔隙度,适合进一步活化以制备活性炭。表征椰壳焦较为合适的方法是:以Ar为分子探针,并采用非定域密度泛函(NLDFT)模型。当校准步骤优先进行时,以N2和Ar为分子探针的吸附测试结果如孔径分布(PSD)和吸附等温线会受到孔隙阻塞的影响,从而错误地描述椰壳焦的孔隙结构。实验结果还表明,273 K下仪器的真空处理可以去除绝大部分残留的He,降低孔隙阻塞的影响。Abstract: To get more insight into the pore structure characterization of nanoporous biomass chars, different probe molecules, models, and calibration steps were used and compared. The coconut shell chars (CSCs) were prepared under a steam atmosphere and characterized using N2, Ar, and CO2 adsorption. The results show that coconut shell chars are suitable for further activation, due to the high carbon content and abundant porosity. Ar adsorption with application of Non-Local Density Functional Theory (NLDFT) model can more accurately characterize the pore structure of CSC. When the calibration step is performed before adsorption measurement, the important results of N2 and Ar adsorption, such as pores size distribution (PSD) and isotherm, are affected by pore blocking, leading to the erroneous understanding of CSC in special applications. Vacuum treatment at 273 K for 1 h after He calibration is enough to remove He, which could reduce effect of pore blocking.
-
Key words:
- adsorption /
- pore structure characterization /
- coconut shell char /
- He calibration /
- pore blocking
-
Table 1 Proximate and ultimate analyses of CSC
Sample Proximate analysis w/% Ultimate analysis w/% Ad Vdaf FCdaf Cdaf Hdaf Ndaf St, d CSC 1.06 3.47 96.53 96.63 0.46 < 0.01 < 0.01 A: ash; V: volatile matter; FC: fixed carbon; C: carbon; H: hydrogen; N: nitrogen; St: total sulfur; d: dry basis;
daf: dry ash-free basisTable 2 SA obtained after application of BET model and NLDFT model (assuming slit-shape pores)
Molecular probe p/p0 Vacuum temperature T/Ka ABET /(m2·g-1) ANLDFT /(m2·g-1) N2 0-0.995 77.4 before 473 593 after 473 596 Ar 0-0.995 77.4 before 453 525 after 459 604 CO2 0-0.03 273 before 438 553 after 438 556 a: samples were evacuated after the He calibration;
before: the He calibration was performed before the adsorption measurement;
after: the He calibration was performed after the adsorption measurement -
[1] KHONDE R, CHAURASIA A. Rice husk gasification in a two-stage fixed-bed gasifier:Production of hydrogen rich syngas and kinetics[J].Int J Hydrogen Energy, 2016, 41(21):8793-8802. doi: 10.1016/j.ijhydene.2016.03.138 [2] MARSH H, RODRÍGUEZ-REINOSO F. Applicability of Activated Carbon[M]. Amsterdam:Elsevier Science, 2006. [3] BENEDETTI V, PATUZZI F, BARATIERI M. Characterization of char from biomass gasification and its similarities with activated carbon in adsorption applications[J]. Appl Energy, 2018, 227:92-99. doi: 10.1016/j.apenergy.2017.08.076 [4] LI W, LIU H F, SONG X X. Multifractal analysis of Hg pore size distributions of tectonically deformed coals[J]. Int J Coal Geol, 2015, 144-145:138-152. doi: 10.1016/j.coal.2015.04.011 [5] ROUQUEROL J, AVNIR D, EVERETT D H, FAIRBRIDGE C, HAYNES M, PERNICONE N, RAMSAY J D F, SING K S W, UNGER K K. Guidelines for the characterization of porous solids[J]. Pure Appl Chem, 2009, 66(8):1739-1758. doi: 10.1016-S0167-2991(08)63059-1/ [6] FIROUZI M, RUPP E C, LIU C W, WILCOX J. Molecular simulation and experimental characterization of the nanoporous structures of coal and gas shale[J]. Int J Coal Geol, 2014, 121(11):123-128. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=1c99afdd79e210f1b7b44bbd8129f36f [7] HAO S X, WEN J, YU XP, CHU W. Effect of the surface oxygen groups on methane adsorption on coals[J]. Appl Surf Sci, 2013, 264:433-442. doi: 10.1016/j.apsusc.2012.10.040 [8] GONZÁLEZ J F, ROMÁN S, GONZÁLEZ-GARCÍA C M, VALENTE NABAIS J M, LUIS ORTIZ A. Porosity development in activated carbons prepared from walnut shells by carbon dioxide or steam activation[J]. Ind Eng Chem Res, 2009, 48(16):7474-7481. doi: 10.1021/ie801848x [9] VARGAS D P, GIRALDO L, MORENO-PIRAJÁN J C. Characterisation of granular activated carbon prepared by activation with CaCl2 by means of gas adsorption and immersion calorimetry[J]. Adsorption, 2016, 22(4/6):717-723. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=8f1a4a2dbdefdcb9043a7328c4b9111e [10] RIOS R V R A, SILVESTRE-ALBERO J, SEPÚLVEDA-ESCRIBANO A, MOLINA-SABIO M, RODRÍGUEZ-REINOSOKINETIC F. Restrictions in the characterization of narrow microporosity in carbon materials[J]. Ieice T Electron, 2011, 94(TENCON):1422-1426. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=7d45718a83856fba888faadbdf3c23a2 [11] LOWELL S, SHIELDS J E, THOMAS M A, THOMMES M. Characterization of porous solids and powders:Surface area, pore size and density[J]. Particle Technol, 2004, 16:1620. doi: 10.1007/978-1-4020-2303-3 [12] TOSO J P, CORNETTE V, YELPO V A, ALEXANDRE DE OLIVEIRA J C, AZEVEDO D C S, LÓPEZ R H. Why the pore geometry model could affect the uniqueness of the PSD in AC characterization[J]. Adsorption, 2016, 22(2):215-222. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=9dce76ec9a749e919473edc4d70020c9 [13] SILVESTRE-ALBERO J, SILVESTRE-ALBERO A, RODRÍGUEZ-REINOSO F, THOMMES M. Physical characterization of activated carbons with narrow microporosity by nitrogen (77.4 K), carbon dioxide (273 K) and argon (87.3 K) adsorption in combination with immersion calorimetry[J]. Carbon, 2012, 50(9):3128-3133. doi: 10.1016/j.carbon.2011.09.005 [14] EISAZADEH A, EISAZADEH H. N2-BET surface area and FESEM studies of lime-stabilized montmorillonitic and kaolinitic soils[J]. Environ Earth Sci, 2015, 74(1):377-384. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=36f559ae1be140a7b9a329f191f37ac7 [15] USTINOV, EUGENE A. Nitrogen adsorption on silica surfaces of nonporous and mesoporous materials[J]. Langmuir, 2008, 24(13):6668-6675. doi: 10.1021/la704011z [16] YANG Z H, GAO Y. BET surface area analysis on microporous materials[J]. Mod Sci Instrum, 2010, (1):97-102. http://d.old.wanfangdata.com.cn/Periodical/xdkxyq201001027 [17] JAGIELLO J, ANIA C O, PARRA J B, JAGIELLO L, PIS J J. Using DFT analysis of adsorption data of multiple gases including H for the comprehensive characterization of microporous carbons[J]. Carbon, 2007, 45(5):1066-1071. doi: 10.1016/j.carbon.2006.12.011 [18] TALU O, MYERS A L. Molecular simulation of adsorption:Gibbs dividing surface and comparison with experiment[J]. AIChE J, 2010, 47(5):1160-1168. doi: 10.1002/aic.690470521/full [19] GUMMA S, TALU O. Gibbs dividing surface and helium adsorption[J]. Adsorption, 2003, 9(1):17-28. http://d.old.wanfangdata.com.cn/NSTLQK/NSTL_QKJJ0224788737/ [20] HERRERA L, FAN C, DO D D, NICHOLSON D. A revisit to the Gibbs dividing surfaces and helium adsorption[J]. Adsorption, 2011, 17(6):955-965. doi: 10.1007/s10450-011-9374-y [21] NEIMARK A V, LIN Y, RAVIKOVITCH P I, THOMMES M. Quenched solid density functional theory and pore size analysis of micro-mesoporous carbons[J]. Carbon, 2009, 47(7):1617-1628. doi: 10.1016/j.carbon.2009.01.050 [22] SILVESTRE-ALBERO J, SILVESTREALBERO A M, LLEWELLYN P L, RODRIGUEZREINOSO F. High-resolution N2 adsorption isotherms at 77.4 K:Critical effect of the He used during calibration[J]. J Phys Chem B, 2013, 117(33):16885-16889. doi: 10.1021/jp405719a [23] HE Q, CAO Y, MIAO Z, REN X F, CHEN J. Estimation of pores distribution in lignite utilizing Hg, H2O, CO2 and N2 as molecular probes[J]. Energy Fuels, 2017, 31(12):13259-13265. doi: 10.1021/acs.energyfuels.7b02131 [24] HUANG S, WU S Y, WU Y Q, GAO J S. Structure characteristics and gasification activity of residual carbon from updraft fixed-bed biomass gasification ash[J]. Energy Convers Manage, 2017, 136:108-118. doi: 10.1016/j.enconman.2016.12.091 [25] DE LANGE M F, VLUGT T J H, GASCON J, KAPTEIJN F. Adsorptive characterization of porous solids:Error analysis guides the way[J]. Microporous Mesoporous Mater, 2014, 200:199-215. doi: 10.1016/j.micromeso.2014.08.048 [26] OCCELLI M L, OLIVIER J P, PETRE A, AUROUX A. Determination of pore size distribution, surface area, and acidity in fluid cracking catalysts (FCCs) from Nonlocal Density Functional Theoretical models of adsorption and from microcalorimetry methods[J]. J Phys Chem B, 2003, 107(17):4128-4136. doi: 10.1021/jp022242m [27] XIONG J, LIU X J, LIANG L X. Experimental study on the pore structure characteristics of the upper ordovician wufeng formation shale in the southwest portion of the sichuan basin[J]. J Nat Gas Sci Eng, 2015, 22:530-539. doi: 10.1016/j.jngse.2015.01.004 [28] FARAMARZI A H, KAGHAZCHI T, EBRAHIM H A, EBRAHIMI A A. A mathematical model for prediction of pore size distribution development during activated carbon preparation[J]. Chem Eng Commun, 2015, 202(2):131-143. doi: 10.1080/00986445.2013.830609 [29] MOELLMER J, CELER E B, LUEBKE R, CAIRNS A J, STAUDT R, EDDAOUDI M, THOMMES M. Insights on adsorption characterization of metalorganic frameworks:A benchmark study on the novel socMOF[J]. Microporous Mesoporous Mater, 2010, 129(3):345-353. doi: 10.1016/j.micromeso.2009.06.014 [30] OKOLO G N, EVERSON R C, NEOMAGUS H W J P, ROBERTS M J, SAKUROVS R. Comparing the porosity and surface areas of coal as measured by gas adsorption, mercury intrusion and SAXS techniques[J]. Fuel, 2015, 141:293-304. doi: 10.1016/j.fuel.2014.10.046 [31] GIBBS J W. The Collected Works of J. W. Gibbs[M]. New York:Longmans and Green, 1928. -





 下载:
下载: