Preparation of amorphous MnOx/TiO2 catalyst and its performance in low temperature NH3-SCR
-
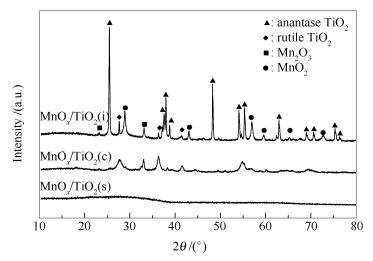
摘要: 采用自发沉积法、共沉淀法及浸渍法制备MnOx/TiO2催化剂,通过XRD、TEM、N2吸附-脱附、XPS、H2-TPR、NH3-TPD等一系列表征手段研究MnOx/TiO2催化剂的结构与性质,并考察MnOx/TiO2催化剂低温NH3-SCR性能。结果表明,自发沉积法制备的MnOx/TiO2(s)催化剂具有完全非晶态结构,Mn和Ti之间存在强相互作用,较共沉淀法制备的MnOx/TiO2(c)及浸渍法制备的MnOx/TiO2(i)表现出更强的氧化还原能力。MnOx/TiO2(s)具有较高的比表面积、较多的表面酸量,有利于NH3的吸附与活化。且表面高浓度的Mn4+离子及吸附氧,有利于将NO氧化为NO2,促进发生"fast-SCR"反应,进而使其表现出优异的低温脱硝性能。MnOx/TiO2(s)催化剂在150 ℃时NO的转化率高达92.8%,在150-350 ℃ NO的转化率保持在90%以上,此外其还具备较强的抗H2O和SO2毒化能力。Abstract: MnOx/TiO2 catalysts were prepared by spontaneous deposition, co-precipitation and impregnation methods, respectively. The structure and properties of the MnOx/TiO2 catalysts were studied by means of XRD, TEM, N2 adsorption-desorption, XPS, H2-TPR and NH3-TPD. The activity of the catalysts in the selective catalytic reduction of NO was investigated. The results showed that the MnOx/TiO2(s) catalyst prepared by the spontaneous deposition method had a completely amorphous structure and a strong interaction between Mn and Ti, showed stronger redox ability than other two catalysts. In addition, the MnOx/TiO2(s) catalyst exhibited larger surface area, more surface acid sites, which were beneficial to NH3 adsorption and activation, as well as the high Mn4+ and adsorbed oxygen content of catalyst surface, which could greatly enhance the activity for NO oxidation to NO2, as a result, facilitating the "fast-SCR" reaction. Thus, a superior denitrification activity was exhibited over MnOx/TiO2(s) catalyst. For MnOx/TiO2(s) catalyst, the conversion of NO reached 92.8% at 150 ℃ and retained over 90% in the range of 150-350 ℃. Moreover, the MnOx/TiO2(s) catalyst also showed strong resistance to H2O and SO2 poisoning.
-
表 1 催化剂的物理化学性质
Table 1 Physical chemical properties of the catalysts

表 2 催化剂的表面原子浓度
Table 2 Surface atomic concentration of the catalysts

-
[1] HU H, CAI S, LI H. HUANG L, SHI L, ZHANG D. Mechanistic aspects of deNOx processing over TiO2 supported Co-Mn oxide catalysts: Structure-activity relationships and in situ DRIFTs analysis[J]. ACS Catal, 2015, 5(10): 6069-6077. doi: 10.1021/acscatal.5b01039 [2] LU X, SONG C, CHANG C C, TENG Y, TONG Z, TANG X. Manganese oxides supported on TiO2-Graphene nanocomposite catalysts for selective catalytic reduction of NOx with NH3 at low temperature[J]. Ind Eng Chem Res, 2014, 53(29): 11601-11610. doi: 10.1021/ie5016969 [3] 朱斌, 费兆阳, 陈献, 汤吉海, 崔咪芬, 乔旭. Al-PILC负载铜铁复合氧化物在NH3选择性催化还原NO中的协同作用[J].燃料化学学报, 2014, 42(9): 1102-1110. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18490.shtmlZHU Bin, FEI Zhao-yang, CHEN Xian, TANG Ji-Hai, CUI Mi-fen, QIAO Xu. Synergetic effect of Cu-Fe composite oxides supported on Al-PILC for SCR of NO with NH3[J]. J Fuel Chem Technol, 2014, 42(9): 1102-1110. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18490.shtml [4] POURKHALIL M, MOGHADDAM A Z, RASHIDI A, TOWFIGHI J, MORTAZAVI Y. Preparation of highly active manganese oxides supported on functionalized MWNTs for low temperature NOx reduction with NH3[J]. Appl Surf Sci, 2013, 279(2): 250-259. http://www.sciencedirect.com/science/article/pii/S0169433213007800 [5] TANG F, XU B, SHI H. The poisoning effect of Na+ and Ca2+ ions doped on the V2O5/TiO2 catalysts for selective catalytic reduction of NO by NH3[J]. Appl Catal B: Environ, 2010, 94(1/2): 71-76. http://www.sciencedirect.com/science/article/pii/S0926337309004305 [6] YANG J, YANG Q, SUN J, LIU Q C, ZHAO D. Effects of mercury oxidation on V2O5-WO3/TiO2 catalyst properties in NH3-SCR process[J]. Catal Commun, 2015, 59: 147-156. http://www.sciencedirect.com/science/article/pii/S1566736714004075 [7] MENG D, ZHAN W, GUO Y, GUO Y, WANG L, LU G. A highly effective catalyst of Sm-MnOx for the NH3-SCR of NOx at low temperature: the promotional role of Sm and its catalytic performance[J]. ACS Catal, 2015, 5(10): 5973-5983. doi: 10.1021/acscatal.5b00747 [8] MU W, ZHU J, ZHANG S, GUO Y, SU L, LI X. Novel proposition on mechanism aspects over Fe-Mn/ZSM-5 catalyst for NH3-SCR of NOx at low temperature: rate and direction of multifunctional electron-transfer-bridge and in-situ DRIFTs analysis[J]. Catal Sci Technol, 2016, 6(20): 7532-7548. doi: 10.1039/C6CY01510G [9] JIANG B Q, LIU Y, WU Z B. Low-temperature selective catalytic reduction of NO on MnOx/TiO2 prepared by different methods[J]. J Hazard Mater, 2009, 162: 1249-1254. doi: 10.1016/j.jhazmat.2008.06.013 [10] ZHANG Y, ZHAO X, XU H, SHEN K, ZHOU C, JIN B, SUN K. Novel ultrasonic-modified MnOx/TiO2 for low-temperature selective catalytic reduction (SCR) of NO with ammonia.[J]. J Colloid Interface Sci, 2011, 361(1): 212-218. http://www.sciencedirect.com/science/article/pii/S0021979711005923 [11] PARK E, KIM M, JUNG H, CHIN S, JURNG J. Effect of sulfur on Mn/Ti catalysts prepared using chemical vapor condensation (CVC) for low-temperature NO reduction[J]. ACS Catal, 2013, 3(7): 1518-1525. doi: 10.1021/cs3007846 [12] 刘俊, 王亮亮, 费兆阳, 陈献, 汤吉海, 崔咪芬, 乔旭.非晶态CeO2@TiO2催化剂的结构、性质及其选择催化还原脱硝性能[J].燃料化学学报, 2016, 44(8): 954-960. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18880.shtmlLIU Jun, WANG Liang-liang, FEI Zhao-yang, CHEN Xian, TANG Ji-hai, CUI Mi-fen, QIAO Xu. Structure and properties of amorphous CeO2@TiO2catalyst and its performance in the selective catalytic reduction of NO with NH3[J]. J Fuel Chem Technol, 2016, 44(8): 954-960. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18880.shtml [13] ZHANG Z P, CHEN L Q, LI Z B. Activity and SO2 resistance of amorphous CeaTiOx catalysts for the selective catalytic reduction of NO with NH3: In-situ DRIFT studies[J]. Catal Sci Technol, 2016, 6(19): 7151-7162. doi: 10.1039/C6CY00475J [14] LI P, XIN Y, LI Q, WANG Z, ZHANG Z, ZHENG L. Ce-Ti amorphous oxides for selective catalytic reduction of NO with NH3: Confirmation of Ce-O-Ti Active Sites[J]. Environ Sci Technol, 2012, 46: 9600-9605. doi: 10.1021/es301661r [15] CHEN X, XU X, FEI Z, XIE, X, Lou, J, TANG J, CUI M, QIAO X. CeO2 nanodots embedded in a porous silica matrix as an active yet durable catalyst for HCl oxidation[J]. Catal Sci Technol, 2016, 6(13): 5116-5123. doi: 10.1039/C5CY02300A [16] FANG Z T, LIN T, XU H D, WU G X, CHEN Y Q. Novel promoting effects of cerium on the activities of NOx reduction by NH3 over TiO2-SiO2-WO3 monolith catalysts[J]. J Rare Earth, 2014, 32(10): 952-959. doi: 10.1016/S1002-0721(14)60168-X [17] PAN S, LUO H, LI L, WEI Z, HUANG B.H2O and SO2 deactivation mechanism of MnOx/MWCNTs for low-temperature SCR of NOx with NH3[J]. J Mol Catal A: Chem, 2013, 377: 154-161. doi: 10.1016/j.molcata.2013.05.009 [18] FANG C, ZHANG D, SHI L, GAO R, LI H, YE L. Highly dispersed CeO2 on carbon nanotubes for selective catalytic reduction of NO with NH3[J]. Catal Sci Technol, 2012, 3(3): 803-811. http://pubs.rsc.org/en/Content/ArticlePDF/2013/CY/c2cy20670f [19] LIU F, HE H, DING Y, ZHANG C. Effect of manganese substitution on the structure and activity of iron titanate catalyst for the selective catalytic reduction of NO with NH3[J]. Appl Catal B: Environ, 2009, 93(1): 3760-3769. http://www.academia.edu/17491828/Effect_of_manganese_substitution_on_the_structure_and_activity_of_iron_titanate_catalyst_for_the_selective_catalytic_reduction_of_NO_with_NH3 [20] SUN P, GUO R T, LIU S M, WANG S X, PAN W G, LI M Y. The enhanced performance of MnOx catalyst for NH3-SCR reaction by the modification with Eu[J]. Appl Catal A: Gen, 2017, 531: 129-138. doi: 10.1016/j.apcata.2016.10.027 [21] 王明洪, 王亮亮, 刘俊, 费兆阳, 陈献, 汤吉海, 崔咪芬, 乔旭.过渡金属对选择性催化还原脱硝CeO2@TiO2催化剂低温活性的促进作用[J].燃料化学学报, 2017, 45(4): 497-504. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract19018.shtmlWANG Ming-hong, WANG Liang-liang, LIU Jun, FEI Zhao-yang, CHEN Xian, TANG Ji-hai, CUI Mi-fen, QIAO Xu. Promoting effect of transition metal on low-temperature deNOx activity of CeO2@TiO2 catalyst for selective catalytic reduction[J]. J Fuel Chem Technol, 2017, 45(4): 497-504. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract19018.shtml [22] ETTIREDDY P R, ETTIREDDY N, MAMEDOV S, BOOLCHAND P, SMIRNIOTIS P G. Surface characterization studies of TiO2 supported manganese oxide catalysts for low temperature SCR of NO with NH3[J]. Appl Catal B: Environ, 2007, 76(1/2): 123-134. doi: 10.1021/ie060484t?src=recsys [23] FANG D, XIE J, HUA H, HU Y, FENG H, FU Z. Identification of MnOx species and Mn valence states in MnOx/TiO2 catalysts for low temperature SCR[J]. Chem Eng J, 2015, 271(1): 23-30. http://www.sciencedirect.com/science/article/pii/S1385894715002818 [24] ARAKAWA K, MATSUDA S, KINOSHITA H. SO2 poisoning mechanism of NOx selective reduction catalysts[J]. Appl Surf Sci, 1997, 121/122: 382-386. doi: 10.1016/S0169-4332(97)00338-3 [25] ARAMEDNDIA M A, BORAU V, JIMENEZ C. Synthesis and characterization of ZrO2 as an acid-base catalyst dehydration-dehydrogenation of propan-2-ol[J]. J Chem Soc, 1997, 93(7):1431-1438. http://pubs.rsc.org/en/Content/ArticleLanding/1997/FT/a606813h#!divAbstract [26] SUN M T, HUANG B C, M J W. Morphological effects of manganese dioxide on catalytic reactions for low-temperature NH3-SCR [J]. Acta Phys Chim Sin, 2016, 32(6): 1501-1510. http://www.whxb.pku.edu.cn/EN/abstract/abstract29439.shtml [27] VÉLEZ R P, ELLMERS I, HUANG H, BENTRUP U, SCHVNEMANN V, GRVNERT W.Identifying active sites for fast NH3-SCR of NO/NO2 mixtures over Fe-ZSM-5 by operando EPR and UV-vis spectroscopy[J]. J Catal, 2014, 316(3): 103-111. http://www.sciencedirect.com/science/article/pii/S0021951714001225 [28] LIU F, HE H, ZHANG C, FENG Z, ZHENG L, XIE Y. Selective catalytic reduction of NO with NH3 over iron titanate catalyst: Catalytic performance and characterization[J]. Appl Catal B: Environ, 2010, 96(3/4): 408-420. http://www.sciencedirect.com/science/article/pii/S0926337310001037 [29] LIU C, SHI J W, GAO C, NIU C M. Manganese oxide-based catalysts for low-temperature selective catalytic reduction of NOx with NH3: A review[J]. Appl Catal A: Gen, 2016, 522: 54-69. doi: 10.1016/j.apcata.2016.04.023 [30] YU J, GUO F, WANG Y, ZHU J, LIU Y, SU F. Sulfur poisoning resistant mesoporous Mn-based catalyst for low-temperature SCR of NO with NH3[J]. Appl Catal B: Environ, 2010, 95(1/2): 160-168. http://www.sciencedirect.com/science/article/pii/S0926337309004913 -





 下载:
下载: