-
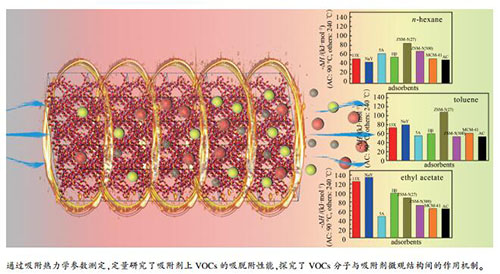
摘要: 采用色谱法与热重(TG)法,测量了正己烷、甲苯和乙酸乙酯在活性炭、5A、NaY、13X、ZSM-5(SiO2/Al2O3=27、300)、Hβ以及MCM-41等吸附剂上不同温度下的吸脱附行为,并基于反相气相色谱法测得的数据,计算了其吸附热力学参数ΔH、ΔS和ΔG,分析了上述VOCs分子与吸附剂之间的作用机制,并借助FT-IR验证了吸附质在分子筛表面的吸附机制。结果表明,上述吸附过程存在物理吸附和化学吸附两种方式,其中,物理吸附的作用力大小与吸附剂的孔径分布和分子直径相关,而化学吸附的作用力大小依赖于分子筛硅铝比和Ca2+、Na+、H+等阳离子及吸附质分子的偶极矩,且强的化学吸附使得部分吸附质分子的脱附温度高于200℃。Abstract: The adsorption and desorption behaviors of n-hexane, toluene and ethyl acetate on activated carbon, 5A, NaY, 13X, ZSM-5 (SiO2/Al2O3=27, 300), Hβ and MCM-41 at different temperatures were investigated by chromatography method and thermogravimetry (TG).And the adsorption thermodynamic parameters (ΔH, ΔS and ΔG) were calculated based on the results obtained by inverse gas chromatography, by which, the interactions between adsorbent and VOC molecules were elucidated. In addition, the adsorption mechanisms of VOC molecules on molecular sieves were confirmed based on FT-IR results. There are two modes of spontaneous adsorption involving physical and chemical adsorptions. The physical adsorption strength is dependent on the pore size distribution of the adsorbent and the molecular diameter of the adsorbate, while the chemical adsorption strength is associated with the Si/Al ratio of the molecular sieve, the cations of Ca2+, Na+, H+, and the dipole moment of the adsorbate molecules. Meanwhile, the presence of strong chemical adsorption makes desorption temperature up to 200℃.
-
Key words:
- desorption temperature /
- VOCs /
- inverse gas chromatography /
- adsorption thermodynamics
-
表 1 VOCs在吸附剂上的吸附热
Table 1 Adsorption heat for typical VOCs on different adsorbents*
Adsorbent -ΔH /(kJ·mol-1) n-hexane toluene ethyl acetate 13X 51.54 73.24 125.39 NaY 44.65 79.61 134.72 5A 62.48 55.34 47.29 Hβ 54.96 59.57 99.42 ZSM-5(27) 84.24 107.92 88.92 ZSM-5(300) 67.38 53.01 72.23 MCM-41 51.55 60.80 65.00 AC 49.33 53.14 64.48 *: adsorption heat obtained at t=90 ℃ for AC and at t=240 ℃ for other adsorbents 表 2 VOCs在吸附剂上的吸附熵
Table 2 Adsorption entropy for typical VOCs on different adsorbents*
Adsorbent -ΔS /(J·mol-1·K-1)* n-hexane toluene ethyl acetate 13X 55.55 91.91 202.83 NaY 54.20 91.08 191.84 5A 76.64 74.53 75.45 Hβ 73.30 78.08 184.79 ZSM-5(27) 128.88 152.15 140.90 ZSM-5(300) 102.02 63.98 100.37 MCM-41 92.05 101.31 103.97 AC 130.13 100.19 134.45 *: adsorption entropy obtained at t=90 ℃ for AC and at t=240 ℃ for other adsorbents 表 3 VOCs在吸附剂上的吉布斯自由能
Table 3 Adsorption Gibbs free energy for typical VOCs on different adsorbents
Adsorbent -ΔG /(kJ·mol-1) n-hexane toluene ethyl acetate 13X 23.04 26.09 21.34 NaY 16.84 32.89 36.31 5A 23.16 17.11 8.58 Hβ 17.35 19.52 4.62 ZSM-5(27) 18.12 29.87 16.64 ZSM-5(300) 15.04 20.19 20.74 MCM-41 4.33 8.83 11.67 AC 2.09 16.77 15.67 *: Gibbs free energy of adsorption obtained at t=90 ℃ for AC and at t=240 ℃ for other adsorbents -
[1] 李长英, 陈明功, 盛楠, 刘启飞, 胡祖和, 方敏, 张涛.挥发性有机物处理技术的特点与发展[J].化工进展, 2016, 35(3):917-925. http://d.old.wanfangdata.com.cn/Periodical/hgjz201603043LI Chang-ying, CHEN Ming-gong, SHENG Nan, LIU Qi-fei, HU Zu-he, FANG Min, ZHANG Tao. The characteristics and development of volatile organic compounds treatment technology[J]. Chem Ind Eng Prog, 2016, 35(3):917-925. http://d.old.wanfangdata.com.cn/Periodical/hgjz201603043 [2] 薛梦婷, 李勇. VOCs在分子筛上吸附性能的研究进展[J].无机盐工业, 2019, 51(5):12-16. http://d.old.wanfangdata.com.cn/Periodical/wjygy201905003XUE Meng-ting, LI Yong. Research progress on adsorption properties of volatile organic compounds on molecular sieves[J]. Inorg Chem Ind, 2019, 51(5):12-16. http://d.old.wanfangdata.com.cn/Periodical/wjygy201905003 [3] 孙健, 戴维杰, 肖伟豪, 郭春梅, 孔繁鑫, 陈进富.挥发性有机物吸附材料研究进展[J].现代化工, 2017, 37(7):58-62. http://d.old.wanfangdata.com.cn/Periodical/xdhg201707014SUN Jian, DAI Wei-jie, XIAO Wei-hao, GUO Chun-mei, KONG Fan-xin, CHEN Jin-fu. Research progress of absorption material for volatile organic compounds[J]. Mod Chem Ind, 2017, 37(7):58-62. http://d.old.wanfangdata.com.cn/Periodical/xdhg201707014 [4] 岳旭, 王胜, 刘旭, 李德意, 王树东, 王建成, 郝兵元.吸附剂动态吸-脱附挥发性有机废气性能研究[J].燃料化学学报, 2020, 48(1):120-128.YUE Xu, WANG Sheng, LIU Xu, LI De-yi, WANG Shu-dong, WANG Jian-cheng, HAO Bing-yuan. Insights on dynamics adsorption/desorption of volatile organic compounds on different adsorbents[J]. J Fuel Chem Technol, 2020, 48(1):120-128. [5] ASKIN A, INEL O G. Evaluation of the heat of adsorption of some n-alkanes on alumina and zeolite by inverse gas chromatography[J]. Sep Sci Technol, 2001, 36(3):381-397. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=10.1081/SS-100102934 [6] CANET X, GILLES F, SU B L, DE WEIRELD G, FRERE M, MOUGIN P. Adsorption of alkanes and aromatic compounds on various faujasites in the Henry domain. 2. Composition effect in X and Y zeolites[J]. J Chem Eng Data, 2007, 52(6):2127-2137. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=0177d59ee5fa240f475db522462f27a4 [7] RUTHVEN D M, KAUL B K. Adsorption of aromatic-hydrocarbons in nax zeolite.1. Equilibrium[J]. Ind Eng Chem Res, 1993, 32(9):2047-2052. http://cn.bing.com/academic/profile?id=5aea60eb9f9a31489cee490ea9d2e7dc&encoded=0&v=paper_preview&mkt=zh-cn [8] DIAZ E, ORDONEZ S, VEGA A, COCA J. Adsorption characterisation of different volatile organic compounds over alumina, zeolites and activated carbon using inverse gas chromatography[J]. J Chromatogr A, 2004, 1049(1/2):139-146. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=3bb0dcca45ff052fef4ea357219c11f5 [9] MILONJIC S K. Surface properties of metal ions modified silicas[J]. Colloid Surf A, 1999, 149(1/3):461-466. http://cn.bing.com/academic/profile?id=b7df73763eaded2e2536097207c629f5&encoded=0&v=paper_preview&mkt=zh-cn [10] SERRANO D P, CALLEJA G, BOTAS J A, GUTIERREZ F J. Characterization of adsorptive and hydrophobic properties of silicalite-1, ZSM-5, TS-1 and Beta zeolites by TPD techniques[J]. Sep Purif Technol, 2007, 54(1):1-9. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=ca05b0a40095064a67b13556ad361ce1 [11] ANGELL C L, HOWELL M V. Infrared spectroscopic investigations of zeolites and adsorbed molecules:III. Aromatic hydrocarbons[J]. J Colloid Interface Sci, 1968, 28(2):279-287. https://www.sciencedirect.com/science/article/pii/0021979768901318 [12] WANG L, SUN B, YANG F H, YANG R T. Effects of aromatics on desulfurization of liquid fuel by π-complexation and carbon adsorbents[J]. Chem Eng Sci, 2012, 73:208-217. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=902da6a43f59fe7712a8a0912867eedc [13] IRA N. LEVINE. Physical Chemistry[M]. 6th ed. New York: McGraw-Hill Education, 2008: 570-575. [14] CANET X, NOKERMAN J, FRERE M. Determination of the Henry constant for zeolite-VOC systems using massic and chromatographic adsorption data[J]. Adsorption, 2005, 11(1):213-216. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=88d8b234b0b8ac7d230e674cc8d77cbc -





 下载:
下载: