Cu-Ni-Al spinel catalyzed methanol steam reforming for hydrogen production: Effect of Al content
-
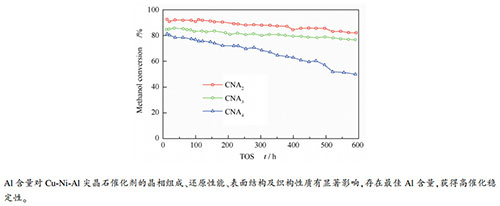
摘要: 采用固相球磨法制备了Al含量不等的Cu-Ni-Al三元尖晶石固溶体催化剂,通过BET、XRD、H2-TPR、XPS表征和催化性能评价,研究了Al含量对Cu-Ni-Al尖晶石的物化性质和甲醇制氢缓释催化性能的影响。结果表明,恒定Cu:Ni(molar ratio)=0.95:0.05,增加Al含量时(Al=2、3、4),所得催化剂的比表面积和孔体积都明显增大,且尖晶石晶胞常数和晶粒尺寸均减小,催化剂也变得难以还原。进一步研究发现,随着Al含量增加,尖晶石Ni2+含量略微增加,但尖晶石Cu2+含量大幅降低,因此,尖晶石结构中Cu2+和Ni2+的总量降低,表明Ni2+的存在抑制了Cu2+进入尖晶石结构。表面分析结果证实,Al含量增加导致催化剂表面由富Cu转变为富Al,表层尖晶石Cu2+含量降低,但仍高于体相含量。评价结果显示,随着Al含量增加,反应初始活性增大,CO选择性降低,但Al过量太多时催化稳定性降低,综合来说,Al=3的催化剂表现出较好的催化性能。结果表明,对于Cu-Ni-Al尖晶石缓释催化剂,存在最佳Al含量,对催化稳定性起到关键作用。
-
关键词:
- Al含量 /
- Cu-Ni-Al尖晶石 /
- 物化性质 /
- 催化性能 /
- 甲醇制氢
Abstract: Cu-Ni-Al ternary spinel solid solution catalysts with different Al content are prepared by the solid-phase ball milling method. The characterizations with XRD, H2-TPR, BET and XPS, and catalytic performance testing are carried out to study the effects of Al content on the physicochemical properties of the Cu-Ni-Al spinels and their sustained release catalytic performances in methanol steam reforming for hydrogen production. Characterization results show a significant increase in the specific surface area and pore volume of the catalysts with increasing the Al content (Al = 2, 3, 4) at a constant Cu/Ni molar ratio of 0.95:0.05. At the same time, both the cell parameters and crystallite sizes of Cu-Ni-Al spinel solid solutions decrease, and the catalysts become difficult to be reduced. Furthermore, the content of spinel Ni2+ increases slightly while the spinel Cu2+ decreases significantly, leading to a declined total content of spinel Cu2+ plus Ni2+. The results also indicate that the presence of Ni2+ inhibits the formation of spinel Cu2+. Surface analysis results show that the increase of Al content transforms the catalyst surface composition from Cu-rich to Al-rich, and the surface spinel Cu2+ decreases, but it is still higher than the spinel bulk. The catalyst testing results show that as the Al content in the catalysts increases, the initial activity increases notably, and the CO selectivity decreases, but too much Al results in an inferior catalytic stability. In general, the catalyst with an Al = 3 shows a better catalytic performance in terms of activity and stability. The results of this paper demonstrate that there is an optimal Al content for the Cu-Ni-Al spinel solid solutions used as the sustained release catalysts, playing a crucial role in obtaining high catalytic stability. -
表 1 催化剂C0.95N0.05Ax(x = 2、3、4)的织构性质
Table 1 Texture properties of C0.95N0.05Ax(x = 2, 3, 4) catalysts
Sample C0.95N0.05A2 C0.95N0.05A3 C0.95N0.05A4 S /(m2·g-1)[a] 30.9 42.2 69.0 v/(cm2·g-1)[b] 0.223 0.291 0.386 d/nm[c] 28.9 27.6 22.4 [a]: catalyst surface areas; [b]: catalyst volumes; [c]: average pore sizes 表 2 C0.95N0.05Ax(x = 2、3、4)及对比样的物化性质
Table 2 Physicochemical properties of C0.95N0.05Ax(x = 2, 3, 4) and the reference samples
Sample CA2 C0.95N0.05A2 C0.95N0.05A3 C0.95N0.05A4 NA2 a/nm[a] 0.8065 0.8063 0.8049 0.8040 0.8045 dspinel/nm[b] 17.1 22.8 11.0 9.0 8.2 X(spinel Cu2+)/%[c] 81.2 77.4 75.8 67.4 - X(non-spinel Cu2+)/%[c] 18.8 17.6 19.2 27.6 - X(spinel Ni2+)/%[c] - 3.5 3.6 4.3 72.4 X(non-spinel Ni2+)/%[c] - 1.5 1.4 0.7 27.6 X(spinel Cu2+ and Ni2+)/%[c] 81.2 80.9 79.4 71.7 72.4 X(hardly-reducible spinel Cu2+)/%[d] 7.2 4.7 18.8 27.0 - [a]: the cell parameters of spinel phases; [b]: the crystallite sizes of spinel phases; [c]: the molar ratio of reducible metal species in catalysts, calculated by peak integrating analysis with H2-TPR profiles; [d]: the hard-reducible spinel Cu2+ species in catalysts 表 3 催化剂C0.95N0.05Ax(x = 2、3、4)的表面性质
Table 3 Surface properties of C0.95N0.05Ax(x = 2, 3, 4) catalysts
Sample C0.95N0.05A2 C0.95N0.05A3 C0.95N0.05A4 Cu/Al (surface)[a] 0.511 0.304 0.202 Cu/Al (bulk)[a] 0.475 0.317 0.238 Cu(s)/Al (spinel surface)[b] 0.477 0.280 0.182 Cu(s)/Al (spinel bulk)[b] 0.387 0.253 0.169 [a]: Cu/Al atomic ratio in the catalyst surface (XPS results) or catalyst bulk (precursors); [b]: Cu(s)/Al atomic ratio in the spinel surface (XPS results) or spinel bulk (H2-TPR results) -
[1] 符冠云.氢能在我国能源转型中的地位和作用[J].中国煤炭, 2019, 45(10):15-21. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgmt201910004FU Guan-yun. The status and role of hydrogen energy in China's energy transformation[J]. China Coal, 2019, 45(10):15-21. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgmt201910004 [2] FASANYA O O, AL-HAJRI R, AHMED O U, MYINT M T Z, ATTA A Y, JIBRIL B Y, DUTTA J. Copper zinc oxide nanocatalysts grown on cordierite substrate for hydrogen production using methanol steam reforming[J]. Int J Hydrogen Energy, 2019, 44(41):22936-22946. doi: 10.1016/j.ijhydene.2019.06.185 [3] MATSUKAT M, UEMIYA S, KIKUCHI E. Copper-alumina spinel catalysts for steam reforming of methanol[J]. Chem Lett, 1988, 17(5):761-764. doi: 10.1246/cl.1988.761 [4] FUKUNAGA T, RYUMON N, ICHIKUNI N, SHIMAZU S. Characterization of CuMn-spinel catalyst for methanol steam reforming[J]. Catal Commun, 2009, 10(14):1800-1803. doi: 10.1016/j.catcom.2009.06.001 [5] MAITI S, DAS D, PAL K, LLORCA J, SOLER L, COLUSSI S, TROVARELLI A, PRIOLKAR K R, SARODE P R, ASAKURA K, SEIKH M M, GAYEN A. Methanol steam reforming behavior of sol-gel synthesized nanodimensional CuxFe1-xAl2O4 hercynites[J]. Appl Catal A:Gen, 2019, 570:73-83. doi: 10.1016/j.apcata.2018.11.011 [6] HWANG B-Y, SAKTHINATHAN S, CHIU T-W. Production of hydrogen from steam reforming of methanol carried out by self-combusted CuCr1-xFexO2 (x=0-1) nanopowders catalyst[J]. Int J Hydrogen Energy, 2019, 44(5):2848-2856. doi: 10.1016/j.ijhydene.2018.12.052 [7] SICKAFUS K E, WILLS.J M. Structure of spinel[J]. J Am Ceram Soc, 1999, 82(12):3279-3292. [8] XI H J, HOU X N, LIU Y J, QING S J, GAO Z X. Cu-Al spinel oxide as an efficient catalyst for methanol steam reforming[J]. Angew Chem Int Ed, 2014, 53(44):11886-11889. doi: 10.1002/anie.201405213 [9] LIU Y J, QING S J, HOU X N, QIN F J, WANG X, GAO Z X, XIANG H W. Cu-Ni-Al spinel oxide as an efficient durable catalyst for methanol steam reforming[J]. ChemCatChem, 2018, 10(24):5698-5706. doi: 10.1002/cctc.201801472 [10] LIU Y J, QING S J, HOU X N, QIN F J, WANG X, GAO Z X, XIANG H W. Temperature dependence of Cu-Al spinel formation and its catalytic performance in methanol steam reforming[J]. Catal Sci Technol, 2017, 7(21):5069-5078. doi: 10.1039/C7CY01236E [11] 刘雅杰, 庆绍军, 侯晓宁, 张磊, 高志贤, 相宏伟. Cu-Al尖晶石的合成及非等温生成动力学分析[J].燃料化学学报, 2020, 48(3):338-348. http://www.ccspublishing.org.cn/article/id/32b9d22d-611e-4472-b517-ec5c891ee8c3LIU Ya-jie, QING Shao-jun, HOU Xiao-ning, ZHANG Lei, GAO Zhi-xian, XIANG Hong-wei.Synthesis of Cu-Al spinels and its non-isothermal formation kinetics analysis[J]. J Fuel Chem Technol, 2020, 48(3):338-348. http://www.ccspublishing.org.cn/article/id/32b9d22d-611e-4472-b517-ec5c891ee8c3 [12] QIN F J, LIU Y J, QING S J, HOU X N, GAO Z X. Cu-Al spinel as a sustained release catalyst for H2 production from methanol steam reforming:Effects of different copper sources[J]. J Fuel Chem Technol, 2017, 45(12) 1481-1488. doi: 10.1016/S1872-5813(17)30065-8 [13] QING S J, HOU X N, LIU Y J, LI L D, WANG X, GAO Z X, FAN W B. Strategic use of CuAlO2 as a sustained release catalyst for production of hydrogen from methanol steam reforming[J]. Chem Commun, 2018, 54(86):12242-12245. doi: 10.1039/C8CC06600K [14] HOU X N, QING S J, LIU Y J, LI L D, GAO Z X, Qin Y. Enhancing effect of MgO modification of Cu-Al spinel oxide catalyst for methanol steam reforming[J]. Int J Hydrogen Energy, 2019, 45(1):477-489. [15] 庆绍军, 侯晓宁, 刘雅杰, 王磊, 李林东, 高志贤. Cu-Ni-Al尖晶石催化甲醇水蒸气重整制氢性能的研究[J].燃料化学学报, 2018, 46(10):1210-1217. http://www.ccspublishing.org.cn/article/id/b14d7a58-9df6-4e70-aff6-8011a66c65eaQING Shao-jun, HOU Xiao-ning, LIU Ya-jie, WANG Lie, LI Lin-dong, GAO Zhi-xian. Catalytic performance of Cu-Ni-Al spinel for methanol steam reforming to hydrogen[J]. J Fuel Chem Technol, 2018, 46(10):1210-1217. http://www.ccspublishing.org.cn/article/id/b14d7a58-9df6-4e70-aff6-8011a66c65ea [16] HOU X N, QIN F J, QING S J, LIU Y J, LI L D, GAO Z X, QIN Y. Probing the existing state of Cu(ii) in a Cu-Al spinel catalyst using N2O decomposition reaction with the aid of conventional characterizations[J]. Catal Sci Technol, 2019, 9(11):2993-3001. doi: 10.1039/C9CY00563C [17] HILL M R, BASTOW T J, CELOTTO S, HILL A J. Integrated study of the calcination cycle from gibbsite to corundum[J]. Chem Mater, 2007, 19:2877-2883. doi: 10.1021/cm070078f [18] MILLER M E, MISTURE S T. Idealizing γ-Al2O3:In situ determination of nonstoichiometric spinel defect structure[J]. J Phys Chem C, 2010, 114:13039-13046. doi: 10.1021/jp102759y [19] RYNKOWSKI J M, PARYJCZAK T, LENIK M. On the nature of oxidic nickel phases in NiO/γ-Al2O3 catalysts[J]. Appl Catal A:Gen, 1993, 106:73-82. doi: 10.1016/0926-860X(93)80156-K [20] MORETTI G, FIERRO G, JACONO M L, PORTA P. Characterization of CuO-ZnO catalysts by X-ray photoelectron spectroscopy:Precursors, calcined and reduced samples[J]. Surf Interface Anal, 1989, 14(6/7):325-336. [21] FIGUEIREDO R T, MARTÍNEZ-ARIAS A, GRANADOS M L, FIERRO J L G. Spectroscopic evidence of Cu-Al interactions in Cu-Zn-Al mixed oxide catalysts used in CO hydrogenation[J]. J Catal, 1998, 178:146-152. doi: 10.1006/jcat.1998.2106 [22] BAHMANPOUR A M, HÉROGUEL F, KILIÇ M, BARANOWSKI C J, SCHOUWINK P, RÖTHLISBERGER U, LUTERBACHER J S, KRÖCHER O. Essential role of oxygen vacancies of Cu-Al and Co-Al spinel oxides in their catalytic activity for the reverse water gas shift reaction[J]. Appl Catal B:Environ, 2020, 266(118669):1-8. [23] ERTL G, HIERL R, KNÖZINGER H, THIELE N, URBACH H P. XPS study of copper aluminate catalysts[J]. Appl Surf Sci, 1980, 5:49-64. doi: 10.1016/0378-5963(80)90117-8 [24] WAGNER C D, DAVIS L E, ZELLER M V, TAYLOR J A, RAYMOND R H, GALE L H. Empirical atomic sensitivity factors for quantitative analysis by electron spectroscopy for chemical analysis[J]. Surf Interface Anal, 1981, 3(5):211-225. doi: 10.1002/sia.740030506 [25] SHIMIZU K-I, MAESHIMA H, YOSHIDA H, SATSUMA A, HATTORI T. Spectroscopic characterisation of Cu-Al2O3 catalysts for selective catalytic reduction of NO with propene[J]. Phys Chem Chem Phys, 2000, 2(10):2435-2439. doi: 10.1039/b000943l [26] NG K T, HERCULE D M. Studies of nickel-tungsten-alumina catalysts by X-ray photoelectron spectroscopy[J]. J Phys Chem, 1976, 80:2094-2102. doi: 10.1021/j100560a009 [27] MATSUMURA Y, TANAKA K, TODE N, YAZAWA T, HARUTA M. Catalytic methanol decomposition to carbon monoxide and hydrogen over nickel supported on silica[J]. J Mol Catal A:Chem, 2000, 152:157-165. doi: 10.1016/S1381-1169(99)00282-4 [28] FURUHASHI H, INAGAKI M, NAKA S. Determination of cation distribution in spinels by X-ray diffraction method[J]. J Inorg Nucl Chem, 1973, 35:3009-3014. doi: 10.1016/0022-1902(73)80531-7 [29] LATHE C, GUSE W, SAALFELD H, HAMBURG, FREIMANN S, RAHMAN S H. Interpretation of σ-Al2O3 real structure by means of X-ray investigations and the videographic method[J]. N Jb Miner Abh, 1999, 174:293-304. -





 下载:
下载: