Effect of calcination temperature on MgAlOx mixed oxides for converting formaldehyde and acetaldehyde to propanal
-
摘要: 采用共沉淀法制备了镁铝水滑石前驱体,通过在不同温度下焙烧得到系列MgAlOx复合氧化物催化剂,采用XRD、TG、N2吸附-脱附、NH3-TPD和CO2-TPD等技术对催化剂的物理和化学性质进行了表征,采用甲醛和乙醛缩合反应对催化剂反应性能进行了评价。结果表明,随着焙烧温度的提高,乙醛转化率以及正丙醛时空收率先增加后减少,C-550催化剂最大,分别达到39.22%和103.86 g/(kg·h),这与催化剂中强碱和强碱数目变化趋势一致。此外,提高催化剂中强碱和强碱数目还会促进副产物甲醇和CO2的生成。
-
关键词:
- 甲醛和乙醛 /
- 正丙醛 /
- MgAlOx复合氧化物 /
- 焙烧温度
Abstract: A series of MgAlOx mixed oxides were prepared by calcination of hydrotalcite materials at various temperatures ranging from 400 to 700℃. The physical and chemical properties of the catalysts were characterized by XRD, TG, N2 adsorption/desorption, NH3-TPD, and CO2-TPD techniques. The catalytic activity was evaluated by the condensation of formaldehyde and acetaldehyde. The results show that as the calcination temperatures increase, both the conversion of acetaldehyde and the space time yield of propanal first increase and then decrease, which shows the same trend with the amount of moderate basic sites, and the C-550 catalyst has the maximum of 39.22% and 103.86 g/(kg·h), respectively. Moreover, the yields of by-products including methanol and CO2 are also significantly related to the moderate and strong basic sites.-
Key words:
- formaldehyde and acetaldehyde /
- propanal /
- MgAlOx mixed oxides /
- calcination temperature
-
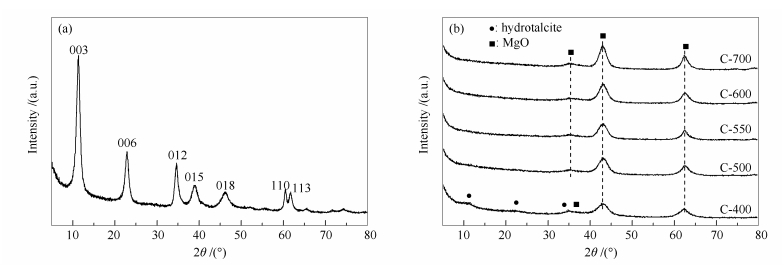
表 1 催化剂的织构参数以及表面酸碱分布
Table 1 Textural properties, acidity, and alkalinity of the samples
Sample ABET /(m2·g-1) vp /(cm·g-1) dp /nm Amount of acid sites /(μmol·g-1)a Amount of basic sites /(μmol·g-1)b total weak moderate total weak moderate strong HT 116.2 0.67 10.86 - - - - - - - C-400 208.6 0.34 6.71 105.68 38.12 67.56 198.76 24.91 141.53 32.32 C-500 226.7 0.49 7.57 171.54 51.69 119.85 267.29 23.69 176.26 67.35 C-550 249.2 0.48 6.53 115.23 38.47 76.76 276.30 6.14 182.93 87.22 C-600 223.8 0.55 8.30 175.43 46.36 129.07 242.11 19.46 162.65 60.00 C-700 165.2 0.13 6.62 135.07 39.65 95.42 213.40 28.35 148.60 36.45 ABET: BET surface area; vp: BJH pore volume; dp: average pore diameter; a: calculated from NH3-TPD; b: calculated from CO2-TPD 表 2 催化剂催化甲醛和乙醛反应的评价
Table 2 Catalytic performance of the catalysts in condensation of formaldehyde and acetaldehyde
Catalyst x/% s/% STY/(g·kg-1·h-1) propanal methanol CO2 isobutanal 1-propanol MF ethanol propanal methanol CO2 C-400 22.12 77.54 9.01 9.60 1.72 0.45 0.75 0.93 60.42 11.61 17.00 C-500 35.69 60.17 18.35 16.32 2.35 0.92 1.05 0.84 95.19 48.04 58.70 C-550 39.22 56.59 19.17 17.80 2.68 1.05 1.98 0.73 103.86 58.23 74.28 C-600 29.27 62.18 15.11 17.11 2.73 0.99 0.87 1.01 77.55 31.19 48.52 C-700 24.21 69.09 13.40 13.00 1.86 0.67 1.06 0.92 65.54 21.04 28.03 reaction conditions: t=260 ℃, p=0.1 MPa, reaction time=5 h, formaldehyde/acetaldehyde (molar ratio)=4, GHSV=1000 h-1, LHSV=2.0 h-1; x: acetaldehyde conversion, s: selectivity, STY: space time yield, MF: methyl formate -
[1] 崔小明.丙醛的生产应用及市场前景[J].杭州化工, 2003, 33(3):17-20. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=schgyfskz200304004CUI Xiao-ming. Production, application, and market prospect pf propaldehyde[J]. Hangzhou Chem Ind, 2003, 33(3):17-20. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=schgyfskz200304004 [2] 李明.丙醛的生产应用及市场前景[J].精细化工原料及中间体, 2005, 23(6):23-29. http://www.cnki.com.cn/Article/CJFDTOTAL-ZJTY2003Z2003.htmLI Ming. Production, application, and market prospect pf propaldehyde[J]. Fine Chem Ind Raw Mater Intermed, 2005, 23(6):23-29. http://www.cnki.com.cn/Article/CJFDTOTAL-ZJTY2003Z2003.htm [3] ZHU X, LOBBAN L L, MALLINSON R G, RESASCO D E. Tailoring the mesopore structure of HZSM-5 to control product distribution in the conversion of propanal[J]. J Catal, 2010, 271(1):88-98. doi: 10.1016/j.jcat.2010.02.004 [4] 张福生.丙醛生产及其应用[J].江苏化工, 1996, 24(4):35-37. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=jshg604.010&dbname=CJFD&dbcode=CJFQZHANG Fu-sheng. Preparation and application of propanal[J]. Jiangsu Chem Ind, 1996, 24(4):35-37. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=jshg604.010&dbname=CJFD&dbcode=CJFQ [5] KAINULAINEN T A, NIEMELA M K, KRAUSE A O I. Ethene hydroformylation on Co/SiO2 catalysts[J]. Catal Lett, 1998, 53(1/2):97-101. doi: 10.1023/A:1019053805618 [6] TRICAS H, DIEBOLT O, VAN LEEUWEN P W N M. Bulky monophosphite ligands for ethene hydroformylation[J]. J Catal, 2013, 298:198-205. doi: 10.1016/j.jcat.2012.11.031 [7] AI M. Formation of acrylaldehyde by vapor-phase aldol condensation 2. Phosphate catalysts[J]. Bull Chem Soc Jpn, 1991, 64(4):1346-1350. doi: 10.1246/bcsj.64.1346 [8] AI M. Formation of acrylaldehyde by vapor-phase aldol condensation 1. Basic oxide catalysts[J]. Bull Chem Soc Jpn, 1991, 64(4):1342-1345. doi: 10.1246/bcsj.64.1342 [9] AZZOUZ A, MESSAD D, NISTOR D, CATRINESCU C, ZVOLINSCHI A, ASAFTEI S. Vapor phase aldol condensation over fully ion-exchanged montmorillonite-rich catalysts[J]. Appl Catal A:Gen, 2003, 241(1/2):1-13. http://www.sciencedirect.com/science/article/pii/S0926860X02005240 [10] DUMITRIU E, HULEA V, CHELARU C, CATRINESCU C, TICHIT D, DURAND R. Influence of the acid-base properties of solid catalysts derived from hydrotalcite-like compounds on the condensation of formaldehyde and acetaldehyde[J]. Appl Catal A:Gen, 1999, 178(2):145-157. doi: 10.1016/S0926-860X(98)00282-8 [11] CAVANI F, TRIFIRO F, VACCARI A. Hydrotalcite-type anionic clays:Preparation, properties and applications[J]. Catal Today, 1991, 11(2):173-301. doi: 10.1016/0920-5861(91)80068-K [12] RAMASAMY K K, GRAY M, JOB H, SANTOSA D, LI X S, DEVARAJ A, KARKAMKAR A, WANG Y. Role of calcination temperature on the hydrotalcite derived MgO-Al2O3 in converting ethanol to butanol[J]. Top Catal, 2016, 59(1):46-54. doi: 10.1007/s11244-015-0504-8 [13] STOSIC D, HOSOGLU F, BENNICI S, TRAVERT A, CAPRON M, DUMEIGNIL F, COUTURIER J L, DUBOIS J L, AUROUX A. Methanol and ethanol reactivity in the presence of hydrotalcites with Mg/Al ratios varying from 2 to 7[J]. Catal Commun, 2017, 89:8-14. http://www.sciencedirect.com/science/article/pii/S1566736716303788 [14] GAO P, LI F, ZHAO N, XIAO F K, WEI W, ZHONG L S, SUN Y H. Influence of modifier (Mn, La, Ce, Zr and Y) on the performance of Cu/Zn/Al catalysts via hydrotalcite-like precursors for CO2 hydrogenation to methanol[J]. Appl Catal A:Gen, 2013, 468:442-452. doi: 10.1016/j.apcata.2013.09.026 [15] 雒京, 李洪广, 赵宁, 王峰, 肖福魁.磺化Salen金属配合物插层水滑石选择性催化氧化甘油制备二羟基丙酮的研究[J].燃料化学学报, 2015, 43(6):677-683. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18640.shtmlLUO Jing, LI Hong-guang, ZHAO Ning, WANG Feng, XIAO Fu-kui. Selective oxidation of glycerol to dihydroxyacetone over layer double hydroxide intercalated with sulfonato-salen metal complexes[J]. J Fuel Chem Technol, 2015, 43(6):677-683. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18640.shtml [16] CANTRELL D G, GILLIE L J, LEE A F, WILSON K. Structure-reactivity correlations in MgAl hydrotalcite catalysts for biodiesel synthesis[J]. App Catal A:Gen, 2005, 287(2):183-190. doi: 10.1016/j.apcata.2005.03.027 [17] GAO P, LI F, ZHAN H J, ZHAO N, XIAO F K, WEI W, ZHONG L S, WANG H, SUN Y H. Influence of Zr on the performance of Cu/Zn/Al/Zr catalysts via hydrotalcite-like precursors for CO2 hydrogenation to methanol[J]. J Catal, 2013, 298:51-60. doi: 10.1016/j.jcat.2012.10.030 [18] 杜亚丽, 谢鲜梅, 吴旭, 胡秋霞, 王志忠. TG-DTA技术在类水滑石化合物热分解研究中的应用[J].应用化工, 2005, 34(9):5-12. http://www.cnki.com.cn/Article/CJFDTOTAL-WJHX200208017.htmDU Ya-li, XIE Xian-mei, WU xu, HU Qiu-xia, WANG Zhi-zhong. TG-DTA technology used in thermal decomposition of hydrotalcite-like compounds[J]. App Chem Ind, 2005, 34(9):5-12. http://www.cnki.com.cn/Article/CJFDTOTAL-WJHX200208017.htm [19] SHEN J Y, TU M, HU C. Structural and surface acid/base properties of hydrotalcite-derived MgAlO oxides calcined at varying temperatures[J]. J Solid State Chem, 1998, 137(2):295-301. doi: 10.1006/jssc.1997.7739 [20] DI COSIMO J I, DIEZ V K, XU M, IGLESIA E, APESTEGUIA C R. Structure and surface and catalytic properties of Mg-Al basic oxides[J]. J Catal, 1998, 178(2):499-510. doi: 10.1006/jcat.1998.2161 [21] 张军, 王秀芝, 赵宁, 肖福魁, 魏伟, 孙予罕.氟修饰的含Ni类水滑石在甲烷部分氧化制合成气中的应用研究[J].燃料化学学报, 2012, 40(4):424-429. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract17921.shtmlZHANG Jun, WANG Xiu-zhi, ZAHO Ning, XIAO Fu-kui, WEI wei, SUN Yu-han. Application of fluorine-modified Ni-Mg-Al hydrotalcite catalyst in the partial oxidation of methane to syngas[J]. J Fuel Chem Technol, 2012, 40(4):424-429. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract17921.shtml [22] LIU Y X, SUN K P, MA H W, XU X L, WANG X L. Zr-incorporated hydrotalcites and their application in the synthesis of isophorone[J]. Catal Commun, 2010, 11(10):880-883. doi: 10.1016/j.catcom.2010.03.014 -





 下载:
下载: