Organic nitrogen promotes stability of metallic catalysts in conversion of bamboo pulp to low carbon polyols
-
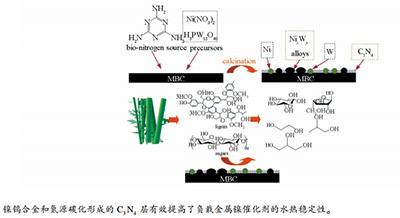
摘要: 采用等体积浸渍法制备了一系列多孔竹炭负载的有机氮掺杂的镍钨催化剂,并将其应用于催化竹浆纤维氢解制C2,3多元醇反应。有机氮源与催化剂前驱体中Ni2+络合,高温煅烧时载体表面碳、氮和金属离子相互作用后生成一定量的C3N4、氮化物和合金物相。通过XRD、XPS和TEM等表征手段分析了催化剂Ni-W/MBC表面物理化学性质与催化活性间的关系。结果表明,除了金属镍、氧化钨物相外,表面还含有Ni-W合金(NiWO4为主);金属粒子表面包围了一层石墨化C3N4物相。XPS分析表明,有机氮源高温分解反应后形成了C3N4物相。在反应条件下,15% Ni-20% W/MBC@M-0.25催化剂得到乙二醇收率为55.8%,而未添加有机氮源的催化剂15% Ni-20% W/MBC获得的乙二醇收率仅为36.9%。催化剂稳定性实验结果表明,Ni-W合金和C3N4物相的形成显著增强了Ni-W/MB催化剂的稳定性,延长了催化剂寿命。Abstract: Herein, the synthesis and performance of a novel and stable catalyst capable of facile hydrolysis of bamboo pulp were reported. Based on adopting complex agent to have a complex reaction with Ni2+ cations, the graphitic g-C3N4 phase and nitride phases were formed eventually. The interaction among metals and C, N atoms was analyzed by XRD and XPS. Some Ni-W alloys (mainly NiWO4 was included) were formed besides metallic Ni0 and tungsten species characterized. Particles on the surface of 15%Ni-20%W/MBC@M-0.25 catalyst exhibited homogeneous distribution and surrounded by disordered C3N4 layer characterized by TEM. Besides, the organic N sources were decomposed and the C3N4 phase with high hydrothermal property was formed simultaneously. For catalytic efficiency, 15%Ni-20%W/MBC@M-0.25 catalyst acquired the highest EG yield of 55.8% compared to 36.9% via 15%Ni-20%W/MBC catalysts. The carbon supports and organic nitrogen sources demonstrated great influence on catalytic efficiency. Catalyst recycle experiments implied that Ni-W/MBC@M-0.25 could remain relative stable under this catalytic reaction condition. The Ni-W alloys and the C3N4 phase were deduced as the main contributors to maintain the catalyst stability.
-
Key words:
- bamboo pulp /
- organic nitrogen /
- metallic catalysts /
- hydrogenolysis /
- polyols
-
Table 1 Physical properties of different supported 15%Ni-20%W catalysts measured by BET
Sample ABET/(m2·g-1) vtotal/(cm3·g-1) Average pore size d/nm MBC@M-0.15 280.7 0.12 7.1 MBC@M-0.20 276.1 0.12 6.1 MBC@M-0.25 271.6 0.12 6.6 MBC@M-0.30 262.9 0.12 5.4 MBC@EDA-0.25 164.2 0.07 5.2 MBC@CAR-0.25 256.1 0.12 9.3 MBC@ARG-0.25 264.1 0.11 4.1 Table 2 Distribution of polyol products from bamboo pulp via nickel-tungsten catalysts a
Entry Catalyst Yield of product/% Conv. x/% EG b Gly 1, 2-PG glucose sor 1 15%Ni-20%W/MBC@M-0.15 29.4 4.1 6.1 5.4 5.1 83 2 15%Ni-20%W/MBC@M-0.20 46.2 3.5 7.6 3.5 4.3 100 3 15%Ni-20%W/MBC@M-0.25 55.8 5.6 10.5 4.8 6.1 100 4 15%Ni-20%W/MBC@M-0.25 b 25.4 3.3 2.1 1.3 trace 76 5 15%Ni-20%W/MBC@M-0.30 51.2 4.7 8.1 3.5 5.3 100 6 15%Ni-20%W/MBC 36.9 6.1 7.2 2.9 3.9 95 7 15%Ni-20%W/AC 21.6 2.3 7.8 3.1 trace 84 8 15%Ni-20%W/SWCNTs c 33.1 8.1 5.6 3.6 4.6 100 9 15%Ni/MBC@M-0.25 10.3 trace 1.2 6.1 1.4 78 10 20%W/MBC@M-0.25 6.5 trace trace 1.2 UD.g 95 11 15%Ni-20%W/MBC@EDA-0.25 d 44.6 4.5 5.3 5.7 4.1 100 12 15%Ni-20%W/MBC@CAR-0.25 e 31.0 5.6 3.2 4.3 0.9 91 13 15%Ni-20%W/MBC@ARG-0.25 f 54.1 6.1 3.0 4.1 3.2 100 a : reaction conditions: bamboo pulp 0.5 g, 5.0 MPa H2, 240 ℃ for 1.5 h; b: feedstock: raw bamboo powder; c: SWNTs: single-walled carbon nanotubes; d: EDA: ethanediamine; e: CAR: carbamide; f: ARG: arginine; g: undetected -
[1] KUMAR A K, SHARMA S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks:A review[J]. Bioresour Bioprocess, 2017, 4(1):7-25. doi: 10.1186/s40643-017-0137-9 [2] JI N, ZHANG T, ZHENG M Y, WANG A Q, WANG H, WANG X D, CHEN J G. Direct catalytic conversion of cellulose into ethylene glycol using nickel-promoted tungsten carbide catalysts[J]. Angew Chem, 2008, 120(44):8638-8641. doi: 10.1002/ange.v120:44 [3] WANG A Q, ZHANG T. One-pot conversion of cellulose to ethylene glycol with multifunctional tungsten-based catalysts[J]. Acc Chem Res, 2013, 46(7):1377-1386. doi: 10.1021/ar3002156 [4] KIM K H, DUTTA T, SUN J, SIMMONS B, SINGH S. Biomass pretreatment using deep eutectic solvents from lignin derived phenols[J]. Green Chem, 2018, 20(4):809-815. doi: 10.1039/C7GC03029K [5] LUO H, ABU-OMAR M M. Lignin extraction and catalytic upgrading from genetically modified poplar[J]. Green Chem, 2018, 20(3):745-753. doi: 10.1039/C7GC03417B [6] DIETRICK K, HERNANDEZ-MEJIA C, VERSCHUREN P, ROTHENBERG G, SHIJU N R. One-pot selective conversion of hemicellulose to xylitol[J]. Org Process Res Dev, 2017, 21(2):165-170. doi: 10.1021/acs.oprd.6b00169 [7] ZHANG Z H, HUBER G W. Catalytic oxidation of carbohydrates into organic acids and furan chemicals[J]. Chem Soc Rev, 2018, 47(4):1351-1390. http://www.ncbi.nlm.nih.gov/pubmed/29297525 [8] XU G, WANG A Q, PANG J F, ZHAO X C, XU J M, LEI N, WANG J, ZHENG M Y, YIN J Z, ZHANG T. Chemocatalytic conversion of cellulosic biomass to methyl glycolate, ethylene glycol, and ethanol[J]. ChemSusChem, 2017, 10(7):1390-1394. doi: 10.1002/cssc.v10.7 [9] DING D Y, ZHOU X, YOU T T, ZHANG X, ZHANG X M, XU F. Exploring the mechanism of high degree of delignification inhibits cellulose conversion efficiency[J]. Carbohydr Polym, 2017, 181(2):931-938. http://www.sciencedirect.com/science/article/pii/S0144861717313437 [10] SHINOHARA N, SUNAGAWA N, TAMURA S, YOKOYAMA R, UEDA M, IGARASHI K, NISHITANI K. The plant cell-wall enzyme AtXTH3 catalyses covalent cross-linking between cellulose and cellooligosaccharide[J]. Sci Rep, 2017, 7(4):46099-46108. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5405413/ [11] ZHENG J, CHOO K, REHMANN L. Xylose removal from lignocellulosic biomass via a twin-screw extruder:The effects of screw configurations and operating conditions[J]. Biomass Bioenergy, 2016, 88(5):10-16. http://www.sciencedirect.com/science/article/pii/S0961953416300617 [12] HAMDY M S, EISSA M A, KESHK S M A S. New catalyst with multiple active sites for selective hydrogenolysis of cellulose to ethylene glycol[J]. Green Chem, 2017, 19(21):5144-5151. doi: 10.1039/C7GC02122D [13] XIAO Z Q, FAN Y, CHENG Y J, ZHANG Q, GE Q, SHA R Y, JI J B, Mao J W. Metal particles supported on SiO2-OH nanosphere:New insight into interactions with metals for cellulose conversion to ethylene glycol[J]. Fuel, 2018, 215(3):406-416. http://www.sciencedirect.com/science/article/pii/S0016236117314898 [14] WANG Y Z, DE S, YAN N. Rational control of nano-scale metal-catalysts for biomass conversion[J]. Chem Commun, 2016, 52(37):6210-6224. doi: 10.1039/C6CC00336B [15] BAEK I G, YOU S J, PARK E D. Direct conversion of cellulose into polyols over Ni/W/SiO2-Al2O3[J]. Bioresour Technol, 2012, 114(3):684-690. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=7d066352839492093ed2a3b75a1dbd6b [16] LIU H L, HUANG Z W, XIA C G, JIA Y Q. Selective hydrogenolysis of xylitol to ethylene glycol and propylene glycol over silica dispersed copper catalysts prepared by a precipitation-gel method[J]. ChemCatChem, 2014, 6(10):2918-2928. doi: 10.1002/cctc.v6.10 [17] YANG Y, ZHANG W, YANG F, BROWN D E, REN Y, LEE S, ZENG D H, GAO Q, ZHANG X. Versatile nickel-tungsten bimetallics/carbon nanofiber catalysts for direct conversion of cellulose to ethylene glycol[J]. Green Chem, 2016, 18(14):3949-3955. doi: 10.1039/C6GC00703A [18] FUKUOKA A, DHEPE P L. Catalytic conversion of cellulose into sugar alcohols[J]. Angew Chem Int Ed, 2006, 118(12):5285-5287. http://www.cnki.com.cn/Article/CJFDTOTAL-JBXG201707004.htm [19] RIBEIRO L S, DELGADO J J, ÍRFAO J J M, PEREIRA M F R. Carbon supported Ru-Ni bimetallic catalysts for the enhanced one-pot conversion of cellulose to sorbitol[J].Appl Catal B:Environ, 2017, 217(15):265-274. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=ae5377def905b680ac11b54bb9c6bf05 [20] HAUSOUL P J C, BEINE A K, NEGHADAR L, PALKOVITS R. Kinetics study of the Ru/C-catalysed hydrogenolysis of polyols-insight into the interactions with the metal surface[J]. Catal Sci Technol, 2017, 7(1):56-63. doi: 10.1039/C6CY02104B [21] ZHENG M Y, WANG A Q, JI N, PANG J F, WANG X D, ZHANG T. Transition metal-tungsten bimetallic catalysts for the conversion of cellulose into ethylene glycol[J]. ChemSusChem, 2010, 3(1):63-66. doi: 10.1002/cssc.v3:1 [22] LI N X, ZHENG Y, WEI LF, TENG H C, ZHOU J C. Metal nanoparticles supported on WO3 nanosheets for highly selective hydrogenolysis of cellulose to ethylene glycol[J]. Green Chem, 2017, 19(3):682-691. doi: 10.1039/C6GC01327A [23] XIAO Z Q, ZHANG Q, CHEN T T, WANG X N, FAN Y, GE Q, ZHAI R, SUN R, JI JB, MAO J W. Heterobimetallic catalysis for lignocellulose to ethylene glycol on nickel tungsten catalysts:Influenced by hydroxy groups[J]. Fuel, 2018, 230(8):332-343. http://www.sciencedirect.com/science/article/pii/S0016236118307488 [24] TAI Z J, ZHANG J Y, WANG A Q, ZHENG M Y, ZHANG T. Temperature-controlled phase-transfer catalysis for ethylene glycol production from cellulose[J]. Chem Commun, 2012, 48(56):7052-7054. doi: 10.1039/c2cc32305b [25] OOMS R, DUSSELIER M, GEBOERS J A, BEECK B O D, VERHAEVEN R, GOBECHIYA E, MARTENS J A, REDL A, SELS B F. Conversion of sugars to ethylene glycol with nickel tungsten carbide in a fed-batch reactor:High productivity and reaction network elucidation[J]. Green Chem, 2014, 16(2):695-707. doi: 10.1039/C3GC41431K [26] WANG J, WEI Z Z, MAO S J, LI H R, WANG Y. Highly uniform Ru nanoparticles over N-doped carbon:pH and temperature-universal hydrogen release from water reduction[J]. Energy Environ Sci, 2018, 11(4):800-806. doi: 10.1039/C7EE03345A [27] CHEN X L, ZHENG J, ZHONG X, JIN Y H, ZHUANG G L, LI X N, DENG S W, WANG J G. Tuning the confinement space of N-carbon shell coated ruthenium nanoparticles:Highly efficient electrocatalysts for hydrogen evolution reaction[J]. Catal Sci Technol, 2017, 7(7):4964-4970. https://www.researchgate.net/publication/319907542_Tuning_The_Confinement_Space_of_N-Carbon_Shell-Coated_Ruthenium_Nanoparticles_Highly_Efficient_Electrocatalysts_for_Hydrogen_Evolution_Reaction [28] DU X D, YI X H, WANG P, DENG J G, WANG C C. Enhanced photocatalytic Cr(Ⅵ) reduction and diclofenac sodium degradation under simulated sunlight irradiation over MIL-100(Fe)/g-C3N4 heterojunctions[J]. Chin J Catal, 2019, 40(1):70-79. http://en.cnki.com.cn/Article_en/CJFDTotal-CHUA201901009.htm [29] HUANG L Y, XU H, LI Y P, LI H M, CHENG X N, XIA J X, XU Y G, CAI G B. Visible-light-induced WO3/g-C3N4 composites with enhanced photocatalytic activity[J]. Dalton Trans, 2013, 42(24):8606-8616. doi: 10.1039/c3dt00115f [30] PAN G Y, MA Y L, MA X X, SUN Y G, LV J M, ZHANG J L. Catalytic hydrogenation of corn stalk into polyol over Ni-W/MCM-41 catalyst[J]. Chem Eng J, 2016, 299(1):386-392. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=102c137e7fd7ee55265f71dd3e165299 [31] YAN C C, LIN L, WANG GX, BAO X H. Transition metal-nitrogen sites for electrochemical carbon dioxide reduction reaction[J]. Chin J Catal, 2019, 40(1):23-37. http://d.old.wanfangdata.com.cn/Periodical/cuihuaxb201901004 [32] XIANG Q J, YU J G, JARONIEC M. Preparation and enhanced visible-light photocatalytic H2-production activity of graphene/C3N4 composites[J]. J Phys Chem C, 2011, 115(15):7355-7363. doi: 10.1021/jp200953k [33] NAZARIAN-SAMANI M, MOBARRA R, KAMALI A R, NAZARIAN-SAMANI M. Structural evolution of nanocrystalline nickel-tungsten alloys upon mechanical alloying with subsequent annealing[J]. Metall Mater Trans A, 2014, 45(1):510-521. doi: 10.1007/s11661-013-1960-z [34] SUDARSANAM P, ZHONG R Y, BOSCH S V D, COMAN S M, PARVULESCU V I, SELS B F. Functionalised heterogeneous catalysts for sustainable biomass valorisation[J]. Chem Soc Rev, 2018, 47(5):8349-8402. http://pubs.rsc.org/en/content/articlelanding/2018/cs/c8cs00410b [35] LI X H, KURASCH S, KAISER U, ANTONIETTI M. Synthesis of monolayer-patched graphene from glucose[J]. Angew Chem Int Ed, 2012, 51(38):9689-9692. doi: 10.1002/anie.v51.38 [36] PUTRO W S, KOJIMA T, HARA T, ICHIKUNI N, SHIMAZU S. Selective hydrogenation of unsaturated carbonyls by Ni-Fe-based alloy catalysts[J]. Catal Sci Technol, 2017, 7(16):3637-3646. doi: 10.1039/C7CY00945C [37] BOONYONGMANEERAT Y, SAENGKIETTIYUT K, SAENGPITAK S, SANGSUK S. Pulse co-electrodeposition and characterization of NiW-WC composite coatings[J]. J Alloys Compd, 2010, 506(1):151-154. doi: 10.1016/j.jallcom.2010.06.162 [38] HONG S H, AHN S H, CHOI J, KIM J Y, KIM H Y, KIM H J, JIANG J H, KIM H, KIM S K. High-activity electrodeposited NiW catalysts for hydrogen evolution in alkaline water electrolysis[J]. Appl Surf Sci, 2015, 349(5):629-635. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=5a89a948961e531dbd48d5b1dd53d40a [39] DI V C, VALENTIN G. Spectroscopic properties of doped and defective semiconducting oxides from hybrid density functional calculations[J]. Acc Chem Res, 2014, 47(11):3233-3241. doi: 10.1021/ar4002944 [40] HUANG Z F, SONG J J, PAN L, ZHANG X W, WANG L, ZOU J J. Tungsten oxides for photocatalysis, electrochemistry and phototherapy[J].Adv Mater, 2015, 27(36):5309-5327. doi: 10.1002/adma.201501217 [41] ZHANG S M, ZHANG H Y, ZHANG W M, YUAN X X, CHEN S L, MA Z F. Induced growth of Fe-Nx active sites using carbon templates[J]. Chin J Catal, 2018, 39(8):1427-1435. doi: 10.1016/S1872-2067(18)63107-9 [42] XIANG Q J, YU J G, JARONIEC M. Preparation and enhanced visible-light photocatalytic H2-production activity of graphene/C3N4 composites[J]. J Phys Chem C, 2011, 115(15):7355-7363. doi: 10.1021/jp200953k [43] YAN S C, LI Z S, ZOU Z G. Photodegradation of rhodamine B and methyl orange over boron-doped g-C3N4 under visible light irradiation[J]. Langmuir, 2010, 26(6):3894-3901. doi: 10.1021/la904023j [44] SUN Y Q, LI C, XU Y X, SHI G Q. Chemically converted graphene as substrate for immobilizing and enhancing the activity of a polymeric catalyst[J]. Chem Commun, 2010, 46(26):4740-4742. doi: 10.1039/c001635g [45] FABICOVICOVA K, LUCAS M, CLAUS P. From microcrystalline cellulose to hard-and softwood-based feedstocks:Their hydrogenolysis to polyols over a highly efficient ruthenium-tungsten catalyst[J]. Green Chem, 2015, 17(15):3075-3083. http://www.researchgate.net/publication/273957745_From_microcrystalline_cellulose_to_hard-_and_softwood-based_feedstocks_their_hydrogenolysis_to_polyols_over_a_highly_efficient_ruthenium-tungsten_catalyst [46] ZHOU L K, WANG A Q, LI C Z, ZHENG M Y, ZHANG T. Selective production of 1, 2-propylene glycol from Jerusalem Artichoke tuber using Ni-W2C/AC catalysts[J]. ChemSusChem 2012, 5(5):932-938. doi: 10.1002/cssc.201100545 [47] ZEMANOVA M, KRIVOSUDSKA M, CHOVANCOVA M, JORIK V. Pulse current electrodeposition and corrosion properties of Ni-W alloy coatings[J]. J Appl Electrochem, 2011, 41(11):1077-1085. doi: 10.1007/s10800-011-0331-y [48] AMANIAMPONG P N, KARAM A, TTINK Q T, XU K, HIRAO H, JEROME F, CHATEL G. Selective and catalyst-free oxidation of D-Glucose to D-Glucuronic acid induced by high-Frequency ultrasound[J]. Sci Rep, 2017, 7(2):40650-40657. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC5233993/ [49] AHMADI M, GUINEL M J F. Electrodeposition and characterization of amorphous-nanocrystalline nickel-tungsten alloys[J]. Microsc Microanal, 2012, 18(S2):1694-1695. doi: 10.1017/S143192761201032X [50] LIU H L, QIN L, WANG X Y, DU C H, SUN D, MENG X C. Hydrolytic hydro-conversion of cellulose to ethylene glycol over bimetallic CNTs-supported NiWB amorphous alloy catalyst[J]. Catal Commun, 2016, 77(14):47-51. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=622a5a3bba91fb2f2c63730db3374699 [51] PEREYMA V Y, KLIMOV O V, PROSVITIN I P, GERASIMOV E Y, YASHNIK S A, NOSKOV A S. Effect of thermal treatment on morphology and catalytic performance of NiW/Al2O3 catalysts prepared using citric acid as chelating agent[J]. Catal Today, 2018, 305(7):162-170. http://www.sciencedirect.com/science/article/pii/S0920586117305023 [52] LI X H, ZHANG J, ZHOU F, ZHANG H L, BAI J, WANG Y J, WANG H Y. Preparation of N-vacancy-doped g-C3N4 with outstanding photocatalytic H2O2 production ability by dielectric barrier discharge plasma treatment[J]. Chin J Catal, 2018, 39(6):1090-1098. doi: 10.1016/S1872-2067(18)63046-3 -





 下载:
下载: