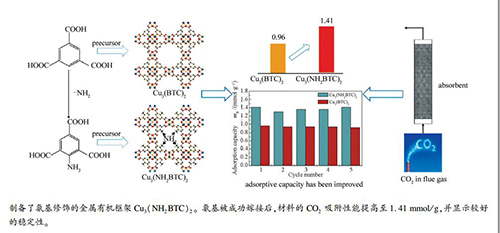
Preparation of metal-organic frameworks Cu3(BTC)2 with amino-functionalization for CO2 adsorption
-
摘要: 首先制备了嫁接氨基的均苯三甲酸,同时以其为原料通过溶剂热法合成了金属有机框架材料Cu3(NH2BTC)2,利用XRD、N2吸附-脱附、热重、红外、原位红外分析等表征手段对吸附剂进行了表征,并通过固定床测量穿透曲线的方法研究其CO2吸附性能。结果表明,氨基被成功引入Cu3(BTC)2骨架中。氨基修饰的Cu3(BTC)2对CO2有着较高的吸附容量,在10 kPa,50 ℃的条件下CO2吸附量为1.41 mmol/g,这源于材料对于CO2同时存在着物理吸附和化学吸附。Abstract: The metal-organic framework of Cu3(NH2BTC)2 was synthesized by solvothermal method with the prepared grafted amine-based trimesic acid as organic ligand. The synthesized adsorbent was characterized by XRD, N2 adsorption-desorption, thermogravimetry, FT-IR and in-situ FT-IR. The performance of the CO2 adsorption was studied by the breakthrough curve based on the fixed-bed reactor. The results showed that the amine groups had been successfully grafted into the skeleton of Cu3(BTC)2. The CO2 adsorption capacity of Cu3(NH2BTC)2 was improved to 1.41 mmol/g at 10 kPa and 50 ℃. The improvement of CO2 uptake might due to the effect of both the physical and chemical adsorption of CO2.
-
Key words:
- amino-functionalized /
- Cu3(BTC)2 /
- CO2 adsorption
1) 本文的英文电子版由Elsevier出版社在ScienceDirect上出版(http://www.sciencedirect.com/science/journal/18725813). -
表 1 两种材料的物性结构参数及在10 kPa,50 ℃下的CO2吸附量
Table 1 Structural properties and CO2 adsorption capacity (10 kPa, 50 ℃) of two samples
Sample BET surface area A/(m2·g-1) Pore volume v/(cm3·g-1) Micropore volume /% (t-plot) Average pore diameter d/nm Adsorption capacity mB /(mmol·g-1) Cu3(BTC)2 735 0.34 91.50 2.00 0.96 Cu3(NH2BTC)2 410 0.16 31.34 4.64 1.41 -
[1] 刘植, 黄少鹏.不同时间尺度下的大气CO2浓度与气候变化[J].第四纪研究, 2015, 35(6):1458-1470. http://d.old.wanfangdata.com.cn/Periodical/dsjyj201506015LIU Zhi, HUANG Shao-peng. Multiple time scales of variations of atmospheric CO2 concentration and global climate[J]. Quat Sci, 2015, 35(6):1458-1470. http://d.old.wanfangdata.com.cn/Periodical/dsjyj201506015 [2] D'ALESSANDRO D M, SMIT B, LONG J R. Carbon dioxide capture:Prospects for new materials[J]. Angew Chem Int Ed, 2010, 49(35):6058-6082. doi: 10.1002/anie.201000431 [3] LEE S-Y, PARK S-J. A review on solid adsorbents for carbon dioxide capture[J]. J Ind Eng Chem, 2015, 23:1-11. doi: 10.1016/j.jiec.2014.09.001 [4] ZHANG Z J, ZHAO Y G, GONG Q H, LI Z, LI J. MOFs for CO2 capture and separation from flue gas mixtures:The effect of multifunctional sites on their adsorption capacity and selectivity[J]. Chem Commun, 2013, 49(7):653-661. doi: 10.1039/C2CC35561B [5] SUMIDA K, ROGOW D L, MASON J S, MCDONALD T M, BLOCH E D, HERM Z R, BAE T H, LONG J R. Carbon dioxide capture in metal-organic frameworks[J]. Chem Rev, 2012, 112(2):724-781. doi: 10.1021/cr2003272 [6] LI J R, SCULLEY J, ZHOU H C. Metal-organic frameworks for separations[J]. Chem Rev, 2012, 112(2):869-932. doi: 10.1021/cr200190s [7] WANG Q M, SHEN D M, BVLOW M, LAU M L, DENG S G, FITCH F R, LEMCOFF N O, SEMANSCIN J. Metallo-organic molecular sieve for gas separation and purification[J]. Microporous Mesoporous Mater, 2002, 55(2):217-230. doi: 10.1016/S1387-1811(02)00405-5 [8] CHUI S S Y, LO S M F, CHARMANT J P H, ORPEN A G, WILLⅡAMS I D. A Chemically functionalizable nanoporous material[Cu3(TMA)2(H2O)3]n[J]. Science, 1999, 283(5405):1148-1150. doi: 10.1126/science.283.5405.1148 [9] KRKLJUS I, HIRSCHER M. Characterizataion of hydrogen/deutetrium adsorption sites in nanoporous Cu-BTC by low-temperature thermal-desorption mass spectroscopy[J]. Microporous Mesoporous Mater, 2011, 142(2/3):725-729. http://www.sciencedirect.com/science/article/pii/S1387181111000370 [10] 朱晨明.基于CO2吸附的分散型MOFs复合材料的制备和吸附性能的研究[D].上海: 上海大学, 2016.ZHU Chen-ming. Preparation and evaluation of hybrid MOFs for CO2 Adsorption[D]. Shanghai: Shanghai University, 2016. [11] YE S, JIANG X, RUAN L W, LIU B, WANG Y M, ZHU J F, QIN L G. Post-combustion CO2 capture with the HKUST-1 and MIL-101(Cr) metal-organic frameworks:Adsorption, separation and regeneration investigations[J]. Microporous Mesoporous Mater, 2013, 179:191-197. doi: 10.1016/j.micromeso.2013.06.007 [12] SU X, BROMBERG L, MARTIS V, SIMEON F, HUQ A, HATTON T A. Postsynthetic functionalization of Mg-MOF-74 with tetraethylenepentamine:Structural characterization and enhanced CO2 adsorption[J]. ACS Appl Mater Inter, 2017, 9(12):11299-11306. doi: 10.1021/acsami.7b02471 [13] MARTÍNEZ F, SANZ R, ORCAJO G, BRIONES D, YÁNGVEZ V. Amino-impregnated MOF materials for CO2 capture at post-combustion conditions[J]. Chem Eng Sci, 2016, 142:55-61. doi: 10.1016/j.ces.2015.11.033 [14] LU W G, WEI Z W, GU Z Y, LIU T F. Tuning the structure and function of metal-organic frameworks via linker design[J]. Chem Soc Rev, 2014, 43(16):5561-5593. doi: 10.1039/C4CS00003J [15] MILLWARD A R, YAGHI O M. Metal-organic frameworks with exceptionally high capacity for storage of carbon dioxide at room temperature[J]. J Am Chem Soc, 2005, 127(51):17998-17999. doi: 10.1021/ja0570032 [16] RADA Z H, ABID H R, SUN H Q, WANG S B. Bifunctionalized metal organic frameworks, UiO-66-NO2-N (N=-NH2, -(OH)2, -(COOH)2), for enhanced adsorption and selectivity of CO2 and N2[J]. J Chem Eng Data, 2015, 60(7):2152-2161. doi: 10.1021/acs.jced.5b00229 [17] DHANKHAR S S, SHARMA N, KUMAR S, KUMAR T J D, NAGARAJA C M. Rational design of a bifunctional, two-fold interpenetrated ZnⅡ-metal-organic framework for selective adsorption of CO2 and efficient aqueous phase sensing of 2, 4, 6-trinitrophenol[J]. Chem Eur J, 2017, 23(64):16204-16212. doi: 10.1002/chem.201703384 [18] ABID H R, RADA Z H, SHANG J, WANG S B. Synthesis, characterization, and CO2 adsorption of three metal-organic frameworks (MOFs):MIL-53, MIL-96, and amino-MIL-53[J]. Polyhedron, 2016, 120:103-111. doi: 10.1016/j.poly.2016.06.034 [19] RUBIN H N, REYNOLDS M M. Functionalization of metal-organic frameworks to achieve controllable wettability[J]. Inorg Chem, 2017, 56(9):5266-5274. doi: 10.1021/acs.inorgchem.7b00373 [20] XIN C L, ZHAN H J, HUANG X, LI H G, ZHAO N, XIAO F K, WEI W, SUN Y H. Effect of various alkaline agents on the size and morphology of nano-sized HKUST-1 for CO2 adsorption[J]. RSC Adv, 2015, 5(35):27901-27911. doi: 10.1039/C5RA03986J [21] 董庆年.红外光谱法[M].北京:石油化学工业出版社, 1977.DONG Qing-nian. Infrared Spectroscopy[M]. Beijing:Petrochemical Industry Press, 1977. [22] PEIKERT K, HOFFMANN F, FRÖBA M. Amino substituted Cu3(btc)2:A new metal-organic framework with a versatile functionality[J]. Chem Commun, 2012, 48(91):11196-11198. doi: 10.1039/c2cc36220a [23] 董寒, 张晓东, 李红欣, 侯扶林, 杨阳, 崔立峰.金属有机骨架材料HKUST-1的制备及其应用进展[J].材料导报A, 2016, 30(12):114-119. http://d.old.wanfangdata.com.cn/Periodical/cldb201623017DONG Han, ZHANG Xiao-dong, LI Hong-xin, HOU Fu-lin, YANG Yang, CUI Li-feng. Progress in preparation of metal organic frameworks HKUST-1 and its application[J]. Mater Rev, 2016, 30(12):114-119. http://d.old.wanfangdata.com.cn/Periodical/cldb201623017 [24] BACSIK Z, AHLSTEN N, ZIADI A, ZHAO G Y, GARCIA-BENNETT A E, MARTÍN-MATUTE B, HEDIN N. Mechanisms and kinetics for sorption of CO2 on bicontinuous mesoporous silica modified with n-Propylamine[J]. Langmuir, 2011, 27(17):11118-11128. doi: 10.1021/la202033p [25] BACSIK Z, RAMBABU A, GARCIA-BENNETT A E, HEDIN N. Temperature-induced uptake of CO2 and formation of carbamates in mesocaged silica modified with n-Propylamines[J]. Langmuir, 2010, 26(12):10013-10024. doi: 10.1021/la1001495 [26] 李勇.有机无极吸附材料的合成及其对CO2吸脱附性能的研究[D].太原: 中国科学院山西煤炭化学研究所, 2013.LI Yong. Synthesis of the organic/inorganic adsorption materials and its adsorption/desorption performance for CO2[D]. Taiyuan: Institute of Coal Chemistry, Chinese Academy of Science, 2013. [27] 辛春玲.微介孔复合材料的制备及其对CO2吸附性能的研究[D].太原: 中国科学院山西煤炭化学研究所, 2015.XIN Chun-ling. Synthesis of mesoporous/microporous composite and its adsorption/desorption performance for CO2[D]. Taiyuan: Institute of Coal Chemistry, Chinese Academy of Science, 2015. -




 下载:
下载: