Study on preparation of high aromatic potential naphtha from pyrolysis heavy oil via hydrocracking
-
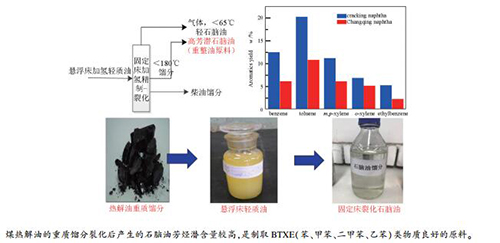
摘要: 以哈密热解焦油重质馏分悬浮床加氢裂化后的轻质油为原料,对其性质进行了分析,轻质油保留了煤的基本单元结构特点,富含芳烃类和环烷烃类化合物,氮含量较高;采用200 mL固定床精制-裂化串联装置,对轻质油原料进行了加氢裂化制取石脑油的研究;反应压力15 MPa下,考察了不同温度对加氢裂化反应的影响。结果表明,适宜的裂化段温度为390℃,此温度下,>180℃馏分转化率为53.69%,氢耗5.13%, < 180℃石脑油收率56.8%,裂化后石脑油主要以C6-9类烃类物质为主,其中,环烷烃含量为71.99%,芳烃含量3.13%,芳潜值70.1;以最佳工艺条件下产出裂化石脑油为原料,进行了催化重整制取BTXE的研究,采用石油系中间基石脑油作为对比,裂化石脑油重整后BTXE类物质总产率为55.85%,较石油基石脑油生成量高25.53%,彰显了煤基油的优势和特点,验证了煤热解重油裂化石脑油是制取BTXE类物质良好的原料。Abstract: The heavy fraction of Hami pyrolysis tar was firstly hydrocracked in a suspended bed reactor, and the as-prepared light product oil was analyzed. The light oil that retains the basic unit structure characteristics of coal and contains a large number of naphthenes and aromatic compounds as well as high nitrogen content was then used to produce naphtha in a 200 mL fixed bed refining-cracking tandem unit. The effect of different temperatures on the hydrocracking reaction was investigated at reaction pressure of 15 MPa. The results show that the optimum cracking temperature is 390℃, at which the conversion of >180℃ fraction is 53.69%, the hydrogen consumption is 5.13%, and the < 180℃ naphtha yield reaches 56.8%. The main components of naphtha are C6-9 hydrocarbons with the naphtha of 71.99%, the naphthene of 3.13% aromatics and the aromatic potential of 70.1. The catalytic reforming of cracked naphtha under optimum conditions to produce BTXE was conducted and compared with that with the middle base naphtha of petroleum series as feed. After reforming, the yield of BTXE from cracking naphtha is 55.85%, 25.53% higher than that from petroleum-based naphtha. The advantages and characteristics of coal-based oil are highlighted. It is verified that naphtha from coal pyrolysis heavy oil hydrocracking is a good raw material for preparing BTXE.
-
Key words:
- pyrolysis heavy oil /
- hydrocracking /
- naphtha /
- naphthene /
- aromatics
-
表 1 煤焦油和煤焦油重油馏分性质
Table 1 Properties of the coal tar and heavy oil
Sample Elemental analysis w/% ρ20a /(kg·m-3) Fraction w/% H C Ob S N saturates aromatics resins aspha ltenes toluene insoluble Coal tar 9.63 83.06 6.4 0.16 0.69 1009 18.07 14.8 55.64 10.38 1.11 Heavy oil 9.34 84.13 5.42 0.14 0.82 1059 15.93 13.89 45.69 23.04 1.94 a: density at 20 ℃;b: by difference 表 2 悬浮床加氢轻质油产物基本性质分析
Table 2 Properties of light fraction products from slurry-bed hydrogenation
Elemental analysis ρ20a /(kg·m-3) Hydrocarbon group composition w/% H w/% C w/% Sb/ (mg·kg-1) Nb/ (mg·kg-1) alkanes naphthenes aromatics polar fraction monocyclic bicyclic tricyclic 11.11 86.32 344 5292 899 32.9 13.68 25.83 21.29 4.18 2.12 fraction distribution v/% IBP 5 10 20 30 40 50 60 70 80 90 EBP t/℃ 100.1 119.4 131.8 178.4 221.2 248.5 277.2 305.4 323.4 347.1 365.9 371.9 a: density at 20 ℃;b: determined by sulfur and nitrogen microanalyzer 表 3 反应条件
Table 3 Reaction condition
No. t/℃ p /MPa Gas-liquid ratio v/v Space velocity /h-1 refining reactor cracking reactor 1 350 370 15.0 1200 0.5 2 350 380 15.0 1200 0.5 3 350 390 15.0 1200 0.5 4 350 400 15.0 1200 0.5 表 4 加氢催化剂性质
Table 4 Properties of hydrogenation catalyst
Catalyst Refining Cracking Composition w/% NiO ≮4.0 ≮7 MoO3 ≮24.0 - WO3 - ≮22 Na2O - 0.08 Carrier γ-Al2O3 Al2O3/SiO2 Shape clover cylinder Surface area A/(m2·g-1) ≮190 ≮170 Mechanical strength /(N·mm-1) ≮18 ≮18 表 5 催化重整催化剂性质
Table 5 Properties of the reforming catalyst
Composition w/% Carrier Specific surface area A/ (m2·g-1) Pore volume v/ (mL· g-1) Mechanical strength /(N· cm-1) Pt Re Cl 0.25 0.25 1.3 γ-Al2O3 >180 0.45-0.55 >100 表 6 加氢裂化物料平衡
Table 6 Material balance of hydro cracking processing
1 2 3 4 Input w/% Light oil 100 100 100 100 DMDS 0.2 0.2 0.2 0.2 H2 3.83 4.21 5.13 5.98 Total 104.03 104.41 105.33 106.18 Output w/% H2S 0.16 0.17 0.17 0.17 NH3 0.64 0.67 0.66 0.66 C1-4 3.27 3.79 4.75 6.89 <180 ℃ 45.47 50.16 56.8 55.44 >180 ℃ 51.98 46.61 39.59 38.8 H2O 2.91 3.01 2.97 2.99 Total 104.03 104.37 105.33 106.18 表 7 加氢裂化后石脑油性质分析
Table 7 Properties of hydro-cracking naphtha
Property ρ20a /(kg·m-3) Bromine value Aromatic potential w/% Elemental analysis w/% H/C (atomic ratio) Trace analysis /(mg·kg-1) C H Nb Sb 1 777 0 69.8 85.93 14.07 1.96 1.9 - 2 776.9 0 73.1 85.32 14.68 2.06 - - 3 770.9 0 70.1 85.01 14.99 2.11 - - 4 767.3 0 66.9 84.95 15.05 2.13 - - a: density at 20 ℃;b: determined by sulfur and nitrogen microanalyzer 表 8 催化重整石脑油性质
Table 8 Naphtha properties of catalytic reforming
Sample Aromatic content w/% IBP-EBP /℃ Group composition w/% nP iP N O A Cracking naphtha 70.1 36.2-180 4.66 21.75 71.99 - 3.28 Changqing naphtha 38.2 28.9-170 29.1 26.13 38.14 0.96 5.67 表 9 重整过程物料平衡
Table 9 Material balance for the reforming experiments
Sample Cracking Changqing Input w/% naphtha 100 100 Output w/% reformed oil 91.12 90.87 H2 3.56 3.69 C1-4 5.32 5.44 Total 100.00 100.00 -
[1] 王建国, 赵晓红.低阶煤清洁高效梯级利用关键技术与示范[J].中国科学院院刊, 2012, 27(3):382-388. doi: 10.3969/j.issn.1000-3045.2012.03.018WANG Jian-guo, ZHAO Xiao-hong. Demonstration of key technologies for clean and efficient utilization of low-rank coal[J]. Bull Chin Acad Sci, 2012, 27(3):382-388. doi: 10.3969/j.issn.1000-3045.2012.03.018 [2] 周剑林, 王永刚, 黄鑫, 张书, 林雄超.低阶煤中含氧官能团分布的研究[J].燃料化学学报, 2013, 41(2):134-138. doi: 10.3969/j.issn.0253-2409.2013.02.002ZHOU Jian-lin, WANG Yong-gang, HUANG Xin, ZHANG Xin, LIN Xiong-chao. Determination of O-containing functional groups distribution in low-rank coals by chemical titration[J]. J Fuel Chem Technol, 2013, 41(2):134-138. doi: 10.3969/j.issn.0253-2409.2013.02.002 [3] KARTHIKEYAN M, WU Z, MUJUMDAR A S. Low-rank coal drying technologies-Current status and new developments[J]. Drying Technol, 2009, 27(3):403-415. doi: 10.1080/07373930802683005 [4] CHESHKO F F, SKRIPCHENKO N P, BANNIKOV L P, KARCHAKOVA V V, PROKHACH E E. Properties of coal tar characterized by slight pyrolysis[J]. Coke Chem, 2014, 57(6):255-259. doi: 10.3103/S1068364X14060027 [5] 何国锋, 戴和武, 金嘉璐, 杜铭华.低温热解煤焦油产率、组成性质与热解温度的关系[J].煤炭学报, 1994, 19(6):591-597. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QK199400684205HE Guo-feng, DAI He-wu, JIN Jia-lu, DU Ming-hua. Relations among yields, characterization of low temperature tars and pyrolysis temperature[J]. J China Coal Soc, 1994, 19(6):591-597. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QK199400684205 [6] SUN Z H, ZHANG W H. Chemical composition and structure characterization of distillation residues of middle-temperature coal tar[J]. Chin J Chem Eng, 2017(6):815-820. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=cjce201706016 [7] FAN X H, FEI Y Q, CHEN L, LI W. Distribution and structural analysis of polycyclic aromatic hydrocarbons abundant in coal tar pitch[J]. Energy Fuels, 2017, 31(5):4694-4704. doi: 10.1021/acs.energyfuels.6b03113 [8] 夏强斌.石脑油型加氢裂化技术及装置设计[J].石油炼制与化工, 2015, 46(6):17-20. doi: 10.3969/j.issn.1005-2399.2015.06.005XIA Qiang-bin. Hydrocracking technology and unit design for naphtha production[J]. Pet Process Petrochem, 2015, 46(6):17-20. doi: 10.3969/j.issn.1005-2399.2015.06.005 [9] PENG C, FANG X C, ZENG R H, GUO R, HAO W Y. Commercial analysis of catalytic hydroprocessing technologies in producing diesel and gasoline by light cycle oil[J]. Catal Today, 2016, 276(1):11-18. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=9e7cce41876587237be592edb75d620c [10] 黄珏, 张德祥, 李洋洋, 高晋生.神华煤液化轻质油的分离、分析及优化利用[J].石油化工, 2010, 39(8):936-940. http://d.old.wanfangdata.com.cn/Periodical/syhg201008019HUANG Jue, ZHANG De-xiang, LI Yang-yang, GAO Jin-sheng. Separation, analysis and optimizing utilization of light oil from shenhua coal liquefaction[J]. Petrochem Technol, 2010, 39(8):936-940. http://d.old.wanfangdata.com.cn/Periodical/syhg201008019 [11] HUANG P, ZHANG X J, MAO X F. Research on the production of aromatic hydrocarbon via hydroreforming a light fraction in direct coal liquefaction oil[J]. Energy Fuels, 2015, 29(1):86-90. doi: 10.1021/ef502146a [12] 佟瑞利, 王永刚, 张旭, 张海永, 戴谨泽, 林雄超, 许德平. P改性NiW/γ-Al2O3的低温焦油芳烃组分加氢性能研究[J].燃料化学学报, 2015, 43(12):1461-1469. doi: 10.3969/j.issn.0253-2409.2015.12.009TONG Rui-li, WANG Yong-gang, ZHANG Xu, ZHANG Hai-yong, DAI Jin-ze, LIN Xiong-chao, XU De-ping. Effect of phosphorus modification on the catalytic properties of NiW/γ-Al2O3 in the hydrogenation of aromatics from coal tar[J]. J Fuel Chem Technol, 2015, 43(12):1461-1469. doi: 10.3969/j.issn.0253-2409.2015.12.009 [13] NIU M L, SUN X H, GAO R, LI D, CUI W G, LI W H. Effect of dephenolization on low temperature coal tar hydrogenation to produce fuel oil[J]. Energy Fuels, 2016, 30(12):10215-10221. doi: 10.1021/acs.energyfuels.6b01985 [14] YUAN Y, LI D, ZHANG L N, ZHU Y H, WANG L, LI W H. Development, status, and prospects of coal tar hydrogenation technology[J]. Energy Technol, 2016, 4(11):1338-1348. doi: 10.1002/ente.201600184 [15] DAI F, GONG M M, LI C S, LI Z X, ZHANG S J. New kinetic model of coal tar hydrogenation process via carbon number component approach[J]. Appl Energy, 2015, 137(C):265-272. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=cbc560684cd877a5e2a770b33eb309cc [16] 孟欣欣, 邱泽刚, 郭兴梅, 李振荣, 胡乃方, 宋毛宁, 赵亮富.不同金属含量Ni-W催化剂的煤焦油加氢脱硫脱氮性能研究[J].燃料化学学报, 2016, 44(5):570-578. doi: 10.3969/j.issn.0253-2409.2016.05.009MENG Xin-xin, QIU Ze-gang, GUO Xing-mei, LI Zhen-rong, HU Nai-fang, SONG Mao-ning, ZHAO Liang-fu. Hydrodenitrogenation and hydrodesulfurization of coal tar on Ni-W catalysts with different metal loadings[J]. J Fuel Chem Technol, 2016, 44(5):570-578. doi: 10.3969/j.issn.0253-2409.2016.05.009 [17] CHEN K, LIU H, XUE Z X, Li H Y, GUO A J, WANG Z X. Co-carbonization of petroleum residue asphaltenes with maltene fractions:Influence on the structure and reactivity of resultant cokes[J]. J Anal Appl Pyrolysis, 2013, 102:131-136. doi: 10.1016/j.jaap.2013.03.005 [18] 黄澎, 张晓静, 毛学锋, 李伟林.神府煤液化油加氢精制过程中硫氮化合物分布的变化[J].燃料化学学报, 2016, 44(1):37-43. doi: 10.3969/j.issn.0253-2409.2016.01.006HUANG Peng, ZHANG Xiao-jing, MAO Xue-feng, LI Wei-lin. Change of sulfur and nitrogen compounds in the direct liquefaction oil from Shenfu coal upon the hydrofining process[J]. J Fuel Chem Technol, 2016, 44(1):37-43. doi: 10.3969/j.issn.0253-2409.2016.01.006 [19] 杨敬一, 周秀欢, 蔡海军, 王红晨.煤焦油和石油基柴油馏分中含氮化合物的分离鉴定[J].石油炼制与化工, 2015, 46(7):107-113. doi: 10.3969/j.issn.1005-2399.2015.07.043YANG Jing-yi, ZHOU Xiu-huan, CAI Hai-jun, WANG Hong-chen. Separation and indentification of nitrogen compounds in diesel fraction of coal tar and petroleum[J]. Pet Process Petrochem, 2015, 46(7):107-113. doi: 10.3969/j.issn.1005-2399.2015.07.043 [20] 吴秀章, 石玉林, 徐春明.煤炭直接液化油品加氢改质中试研究[J].石油学报(石油加工), 2009, 25(2):156-161. doi: 10.3969/j.issn.1001-8719.2009.02.004WU Xiu-zhang, SHI Yu-lin, XU Chun-ming. Hydro-upgrading pilot test of direct coal liquefaction effluent[J]. Acta Pet Sin(Pet Process Sect), 2009, 25(2):156-161. doi: 10.3969/j.issn.1001-8719.2009.02.004 [21] 孙国权, 姚春雷, 全辉, 张志银.中低温煤焦油加氢生产清洁燃料技术的开发及工业应用[J].石油炼制与化工, 2015, 46(8):12-17. doi: 10.3969/j.issn.1005-2399.2015.08.003SUN Guo-quan, YAO Chun-lei, QUAN Hui, ZHANG Zhi-yin. Development and commercialization of clean fuels production technology from medium and low temperature coal tars[J]. Pet Process Petrochem, 2015, 46(8):12-17. doi: 10.3969/j.issn.1005-2399.2015.08.003 [22] 李立权.加氢裂化装置工艺计算与技术分析[M].北京:中国石化出版社, 2009.LI Li-quan. Process Calculation and Technical Analysis of Hydro Cracking Unit[M]. Beijing:China Petrochemical Press, 2009. [23] WANG J, ZHAO S Q, XU C, CHUNG K H. Properties correlations and characterization of athabasca oil sands-derived synthetic crude oil[J]. Pet Sci, 2007, 4(3):84-90. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=sykx-e200703014 [24] 王雷, 李会鹏.炼油工艺学[M].北京:中国石化出版社, 2011.WANG Lei, LI Hui-peng. Petroleum Refining Technology[M]. Beijing:China Petrochemical Press, 2011. -





 下载:
下载: