In-situ electrodeposited flower-like NiFeOxHy/rGO on nickel foam for oxygen evolution reaction
-
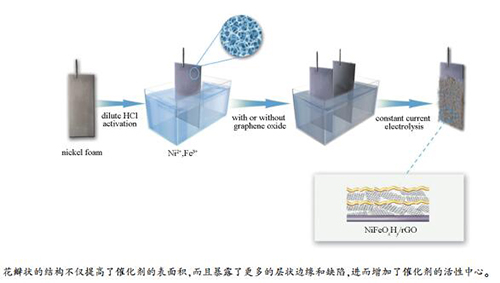
摘要: 开发碱性体系的高效低成本析氧电催化剂是由可再生能源转化制氢的关键。本研究通过在泡沫Ni基底上原位电化学沉积的方法制备了花瓣状NiFeOxHy和NiFeOxHy/rGO复合催化剂用于析氧反应。花瓣状的结构不仅明显提高了催化剂的比表面积,而且暴露了更多的层状边缘和缺陷,进而增加了催化剂的活性中心。还原氧化石墨烯的加入进一步提升了催化剂的电导和析氧电催化性能,通过优化NiFeOxHy/rGO在1 mol/L KOH溶液中的析氧性能为:过电位200 mV(10 mA/cm2)、Tafel斜率29.11 mV/decade,并且保持了较好的稳定性。
-
关键词:
- 析氧反应 /
- NiFeOxHy/rGO /
- 电催化 /
- 花瓣状
Abstract: Developing cost-effective electrocatalysts for oxygen evolution reaction (OER) in basic media is critical to hydrogen production from renewable energy. Herein, in-situ electrodeposited flower-like NiFeOxHy and NiFeOxHy/rGO composite electrocatalysts on Ni foam for OER are reported. The active sites of the flower-like electrocatalysts are increased significantly due to the enhanced NiFeOxHy surface areas and numerous exposed layered edges and edge defects. Reduced graphene oxide (rGO) has been introduced to fabricate NiFeOxHy/rGO composite film, further improving the conductivity and OER performance of the flower-like NiFeOxHy. The optimized NiFeOxHy/rGO exhibits superior OER performance with a Tafel slope of 29.11 mV/decade, an overpotential of 200 mV at 10 mA/cm2 in 1 mol/L KOH and favorable long-term stability.-
Key words:
- oxygen evolution reactions /
- NiFeOxHy/rGO /
- electrocatalysis /
- flower-like
-
Table 1 ICP results of NiFeOxHy and NiFeOxHy/rGO composite electrocatalysts
Entry Elements w/% Composition NiFeOxHy Ni
Fe53.2%
46.8%Ni52Fe48 NiFeOxHy/rGO Ni
Fe53.3%
46.7%Ni52Fe48 Ni1Fe2OxHy/rGO Ni
Fe34.4%
65.6%Ni34Fe66 Ni2Fe1OxHy/rGO Ni
Fe70.1%
29.9%Ni69 Fe31 -
[1] SHERIF S A, BARBIR F, VEZIROGLU T N. Wind energy and the hydrogen economy-review of the technology[J]. Sol Energy, 2005, 78(5):647-660. [2] TRONCOSO E, NEWBOROUGH M. Implementation and control of electrolysers to achieve high penetrations of renewable power[J]. Int J Hydrogen Energy, 2007, 32(13):2253-2268. doi: 10.1016/j.ijhydene.2007.02.034 [3] WANG J, CUI W, LIU Q, XING Z, ASIRI A M, SUN X. Recent progress in cobalt-based heterogeneous catalysts for electrochemical water splitting[J]. Adv Mater, 2016, 28:215-230 doi: 10.1002/adma.201502696 [4] DENG X, TVYSUVZ H. Cobalt-oxide-based materials as water oxidation catalyst:Recent progress and challenges[J]. ACS Catal, 2014, 4(10):3701-3714. doi: 10.1021/cs500713d [5] SUEN N, HUNG S, QUAN Q, ZHANG N, XU Y, CHEN H M. Electrocatalysis for the oxygen evolution reaction:recent development and future perspectives[J]. Chem Soc Rev, 2016, 46:337-365. [6] LEE D U, XU P, CANO Z P, KASHKOOLI A G, PARK M G, CHEN Z. Recent progress and perspectives on bi-functional oxygen electrocatalysts for advanced rechargeable metal-air batteries[J]. J Mater Chem A, 2016, 4:7107-7134. doi: 10.1039/C6TA00173D [7] REIER T, OEZASLAN M, STRASSER P. Electrocatalytic oxygen evolution reaction (OER) on Ru, Ir, and Pt catalysts:A comparative study of nanoparticles and bulk materials[J]. ACS Catal, 2012, 2(8):1765-1772. doi: 10.1021/cs3003098 [8] YOUN D H, PARK Y B, KIM J Y, MAGESH G, JANG Y, LEE J S. One-pot synthesis of NiFe layered double hydroxide/reduced graphene oxide composite as an efficient electrocatalyst for electrochemical and photoelectrochemical water oxidation[J]. J Power Sources, 2015, 294:437-443 doi: 10.1016/j.jpowsour.2015.06.098 [9] CHEN Y, RUI K, ZHU J, DOU S X, SUN W. Recent progress on nickel-based oxide/(oxy)hydroxide electrocatalysts for the oxygen evolution reaction[J]. Chem Eur J, 2019, 25(3):703-713. doi: 10.1002/chem.201802068 [10] GONG M AND DAI H. A mini review of NiFe-based materials as highly active oxygen evolution reaction electrocatalysts[J]. Nano Res, 2015, 8(1):23-39. [11] ZHU K, ZHU X. YANG W. Application of in situ techniques for the characterization of NiFe-based oxygen evolution reaction (OER) electrocatalysts[J]. Angew Chem Int Ed, 2019, 58(5):1252-1265. doi: 10.1002/anie.201802923 [12] CHEN J Y, DANG L, LIANG H, BI W, GERKEN J B, JIN S, ALP E E, STAHL S S. Operando analysis of NiFe and Fe oxyhydroxide electrocatalysts for water oxidation:Detection of Fe4+ by mössbauer spectroscopy[J]. J Am Chem Soc, 2015, 137(48):15090-15093. doi: 10.1021/jacs.5b10699 [13] AHN H S, BARD A J. Surface interrogation scanning electrochemical microscopy of Ni1-xFexOOH (0 < x < 0.27) oxygen evolving catalyst:Kinetics of the "fast" iron sites[J]. J Am Chem Soc, 2016, 138(1):313-318. doi: 10.1021/jacs.5b10977 [14] GÖRLIN M, ARAÚJO J F, SCHMIES H, BERNSMEIER D, DRESP S, GLIECH M, JUSYS Z, CHERNEV P, KRAEHNERT R, DAU H, STRASSER P. Tracking catalyst redox states and reaction dynamics in Ni-Fe oxyhydroxide oxygen evolution reaction (OER) electrocatalysts:the role of catalyst support and electrolyte pH[J]. J Am Chem Soc, 2017, 139(5):2070-2082. doi: 10.1021/jacs.6b12250 [15] ZHOU Q, CHEN Y, ZHAO G, LIN Y, YU Z, XU X, WANG X, LIU H, SUN W, DOU S X. Active site-enriched iron-doped nickel/cobalt hydroxide nanosheets for enhanced oxygen evolution reaction[J]. ACS Catal, 2018, 8(6):5382-5390. doi: 10.1021/acscatal.8b01332 [16] YAN K, LAFLEUR T, CHAI J, JARVIS C. Facile synthesis of thin NiFe-layered double hydroxides nanosheets efficient for oxygen evolution[J]. Electrochem Commun, 2015, 62:24-28. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=708f7cba7285bd4eed016cace95417bc [17] LU X, ZHAO C. Electrodeposition of hierarchically structured three-dimensional nickel-iron electrodes for efficient oxygen evolution at high current densities[J]. Nat Commun, 2015, 6:6616. doi: 10.1038/ncomms7616 [18] MORALES-GUIO C G, LIARDET L, HU X. Oxidatively electrodeposited thin film transition metal (oxy)hydroxides as oxygen evolution catalysts[J]. J Am Chem Soc, 2016, 138(28):8946-8957. doi: 10.1021/jacs.6b05196 [19] TRZESNIEWSKI B J, DIAZ-MORALES O, VERMAAS D A, LONGO A, BRAS W, KOPER M, SMITH W A. In situ observation of active oxygen species in Fe-Containing Ni-based oxygen evolution catalysts:the effect of pH on electrochemical activity[J]. J Am Chem Soc, 2015, 137(48):15112-15121. [20] FRIEBEL D, LOUIE M W, BAJDICH M, SANWALD K E, CAI Y, WISE A M, CHENG M, SOKARAS D, WENG T, ALONSO-MORI R, DAVIS R C, BARGAR J R, NORSKOV J K, NILSSON A, BELL A T. Identification of highly active Fe sites in (Ni, Fe)OOH for electrocatalytic water splitting[J]. J Am Chem Soc, 2015, 137(3):1305-1313. doi: 10.1021/ja511559d [21] SHAO Y, WANG J, ENGELHARD M, WANG C, LIN Y. Facile and controllable electrochemical reduction of graphene oxide and its applications[J]. J Mater Chem, 2010, 20:743-748. doi: 10.1039/B917975E [22] GUO H, WANG X, QIAN Q, WANG F, XIA X. A green approach to the synthesis of graphene nanosheets[J]. ACS Nano, 2009, 3(9):2653-2659. doi: 10.1021/nn900227d [23] HUMMERS W S, OFFEMAN R E. Preparation of graphitic oxide[J]. J Am Chem Soc, 1958, 80(6):1339-1339. doi: 10.1021/ja01539a017 [24] RONG F, ZHAO J, YANG Q, LI C. Nanostructured hybrid NiFeOOH/CNT electrocatalysts for oxygen evolution reaction with low overpotential[J]. RSC Adv, 2016, 6:74536-74544 doi: 10.1039/C6RA16450A [25] LIU R, WANG Y, LIU D, ZOU Y, WANG S. Water-plasma-enabled exfoliation of ultrathin layered double hydroxide nanosheets with multivacancies for water oxidation[J]. Adv Mater, 2017, 29:1701546. doi: 10.1002/adma.201701546 [26] ZHANG Y, LU J. A mild and efficient biomimetic synthesis of rodlike hydroxyapatite particles with a high aspect ratio using polyvinylpyrrolidone as capping agent[J]. Cryst Growth Des, 2008, 8(7):2101-2107. doi: 10.1021/cg060880e -





 下载:
下载: