Effect of calcination temperature on the structure and properties of Raney-Ni catalyst for hydrogenation of 1, 4-butenediol
-
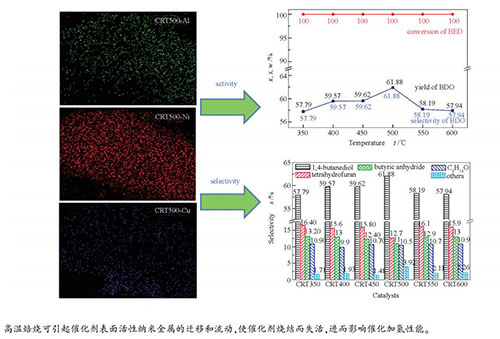
摘要: 在Ni-Al合金粉上浸渍硝酸铜溶液,经不同温度焙烧得到Cu改性Ni-Al合金粉,采用质量分数10% NaOH溶液浸渍上述改性合金粉得Cu/Raney-Ni催化剂。采用EDX、XRD、N2吸附-脱附、TEM和NH3-TPD等手段表征了Ni-Al合金粉及相应Raney-Ni催化剂的元素含量、晶体结构、孔结构特征、表面形貌和表面酸碱性,并以1,4-丁烯二醇加氢制1,4-丁二醇为探针反应,考察了焙烧温度对Raney-Ni催化剂加氢性能的影响。表征分析表明,焙烧温度500 ℃所制备的CRT500催化剂比表面积较大,为64.96 m2/g;弱酸中心比例较高,达81.2%。结果表明,焙烧温度升高,BED可实现完全转化,BDO选择性和收率均先升高后降低。其中,CRT500加氢性能较好,BED转化率为100.00%,BDO选择性为61.88%。进一步升高焙烧温度,催化剂RCT550和RCT600的BDO选择性和收率反而降低,这是由于高温下催化剂易发生团聚或烧结。结合催化剂表征可知,CRT500具有较好的加氢性能,这与该催化剂具有合适的Ni/Al物质的量比(3.84)、弱酸中心所占比例较大和活性组分Ni分散性好等因素有较大关联。
-
关键词:
- 浸渍法 /
- Raney-Ni催化剂 /
- 1, 4-丁烯二醇 /
- 焙烧温度
Abstract: Ni-Al alloy powder was impregnated with copper nitrate solution, and calcined at different temperatures to obtain Cu-modified Ni-Al alloy powder. The modified alloy powder was leached with a 10% (mass ratio) NaOH solution to obtain the Cu/Raney-Ni catalyst. Elemental composition, crystal structure, pore structure, surface morphology and surface acidity of the Ni-Al alloy powder and corresponding Raney-Ni catalysts were characterized by EDX, XRD, N2 adsorption-desorption, TEM and NH3-TPD. The hydrogenation performance of the Raney-Ni catalysts were evaluated using the reaction of 1, 4-butenediol (BED) hydrogenation to 1, 4-butanediol (BDO) as the probe reaction. The characteristic results showed that the CRT500 catalyst prepared at the calcination temperature of 500 ℃ presented larger specific surface area of 64.96 m2/g, and the proportion of weak acid sites was high of 81.2%. The reaction results proposed that the reactant of BED could be completely converted, and the selectivity and yield of BDO increased firstly and then decreased as the calcination temperature increased. The CRT500 catalyst presented good hydrogenation performance, with BED conversion of 100.00%, BDO selectivity of 61.88%, while the BDO selectivity of the RCT550 and RCT600 were lower, which might be due to the agglomeration or sintering of the catalyst at higher calcination temperature. The CRT500 catalyst showed excellent hydrogenation performance, which might be attributed to the appropriate molar ratio of Ni/Al (3.84), the large proportion of weak acid sites and good dispersion of active component Ni.-
Key words:
- impregnation /
- Raney-Ni catalyst /
- 1, 4-butenediol /
- calcination temperature
-
图 6 催化剂的EDX-Mapping照片
Figure 6 EDX-Mapping diagrams of the catalysts
(a): CRT350-Al; (b): CRT350-Ni; (c): CRT350-Cu; (d): CRT400-Al; (e): CRT400-Ni; (f): CRT400-Cu; (g): CRT450-Al; (h): CRT450-Ni; (i): CRT450-Cu; (j): CRT500-Al; (k): CRT500-Ni; (l): CRT500-Cu; (m): CRT550-Al; (n): CRT550-Ni; (o): CRT550-Cu; (p): CRT600-Al; (q): CRT600-Ni; (r): CRT600-Cu
表 1 Ni2Al3相的晶粒粒径
Table 1 Grain size of Ni2Al3
Sample Size of Ni2Al3 d/nm RT350 1.059 RT400 1.027 RT450 0.886 RT500 0.876 RT550 0.863 RT600 0.678 表 2 焙烧后试样的元素含量
Table 2 Elemental contents of the catalysts calcined at different temperatures
Catalyst Content w/% O Al Ni Cu RT350 1.33 44.13 43.36 11.18 RT400 0.14 52.42 37.20 10.24 RT450 0.14 52.42 38.92 8.53 RT500 0.13 43.17 49.90 6.80 RT550 0.15 45.25 49.04 5.55 RT600 0.19 56.81 38.54 4.46 表 3 合金粉的比表面积、孔体积和平均孔径
Table 3 Specific surface area, pore volume, average pore diameter of the alloy powder
Sample Specific surface area A/(m2·g-1) Pore volume v/(cm3·g-1) Average pore diameter d/nm RT350 21.1 0.025 4.92 RT400 14.37 0.019 5.36 RT450 33.44 0.019 3.68 RT500 13.49 0.020 5.59 RT550 10.44 0.016 5.61 RT600 11.06 0.018 5.88 表 4 催化剂的元素含量
Table 4 Elemental contents of the catalyst samples
Sample Content w/% Ni/Al
(mol ratio)Al Ni Cu CRT350 12.07 75.60 12.33 2.88 CRT400 12.78 75.28 11.94 2.71 CRT450 9.91 80.48 9.61 3.76 CRT500 9.68 80.83 9.49 3.84 CRT550 8.39 81.78 9.83 4.48 CRT600 8.58 82.56 8.86 4.42 表 5 Cu相和Ni相的晶粒粒径
Table 5 Crystal grain size of Ni and Cu
Sample Pore size d /nm Cu Ni CRT350 0.486 0.229 CRT400 0.434 0.167 CRT450 0.610 0.217 CRT500 0.461 0.179 CRT550 0.466 0.183 CRT600 0.498 0.196 表 6 催化剂的比表面积、孔体积和平均孔径
Table 6 Specific surface area, pore volume, average pore diameter of the catalysts
Sample Specific surface area A/(m2·g-1) Pore volume v/(cm3·g-1) Average pore diameter d/nm CRT350 33.75 0.047 5.44 CRT400 51.54 0.063 4.82 CRT450 62.18 0.060 4.13 CRT500 64.96 0.075 4.85 CRT550 52.27 0.063 4.88 CRT600 57.83 0.065 4.73 表 7 弱酸和中强酸峰面积所占比例
Table 7 Proportion of weak acid peak area and medium acid peak area
Sample Peak area proportion
of the weak acid /%Peak area proportion
of the medium acid /%CRT350 67.8 32.2 CRT400 72.5 27.5 CRT450 79.5 20.5 CRT500 81.2 18.8 CRT550 75.3 24.7 CRT600 75.7 24.3 -
[1] WANG J, JAIN R C, SHEN X L, CHENG M Y, JAMES C L, YUAN Q P, YAN Y J. Rational engineering of diol dehydratase enables 1, 4-butanediol biosynthesis from xylose[J]. Metab Eng, 2017, 40:148-156. doi: 10.1016/j.ymben.2017.02.003 [2] DEMATTEIS M, PENNEL L, MALLARET M. Current knowledge on gamma-hydroxybutyric acid (GHB), gamma-butyrolactone (GBL) and 1, 4-butanediol (1, 4-BD)[J]. Rev Prat, 2012, 62(5):669-672. http://www.ncbi.nlm.nih.gov/pubmed/22730800 [3] PYATNITSYNA E V, EL'CHANINOV I M, EL' CHANINOV M M. Chemical method for removal of impurities impairing the quality of commercial 1, 4-butanediol produced by the reppe method[J]. Russ J Appl Chem, 2014, 87(1):104-107. doi: 10.1134/S1070427214010157 [4] 莫文龙, 郑霜, 马亚亚, 马凤云, 艾沙·努拉洪, 席龙飞.制备方法对1, 4-丁炔二醇加氢Ni-Al2O3催化剂性能的影响[J].石油学报(石油加工), 2019, 35(2):252-260. doi: 10.3969/j.issn.1001-8719.2019.02.005MO Wen-long, ZHENG Shuang, MA Ya-ya, MA Feng-yun, AISHA·Nu-la-hong, XI Long-fei. Influence of preparation methods on the performance of Ni-Al2O3 catalyst for hydrogenation of 1, 4-butynediol[J]. Acta Pet Sin (Pet Process Sect), 2019, 35(2):252-260. doi: 10.3969/j.issn.1001-8719.2019.02.005 [5] LI H T, XU Y L, GAO C G, WANG Y Z. Structural and textural evolution of Ni/γ-Al2O3 catalyst under hydrothermal conditions[J]. Catal Today, 2010, 158:475-480 doi: 10.1016/j.cattod.2010.07.015 [6] LI H T, ZHAO Y X, GAO C G, WANG Y Z, SUN Z J, LIANG X Y. Study on deactivation of Ni/Al2O3 catalyst for liquid phase hydrogenation of crude 1, 4-butanediol aqueous solution[J]. Chem Eng J, 2012, 181(1):501-507. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=41d404b3577c13c0f4004ab5d56ea859 [7] ANANDA S A, YEN M L, ANTHONY D F, TONY L G, BERNARD W. Conversion of levulinic acid and cellulose to γ-valerolactone over Raney-Ni catalyst using formic acid as a hydrogen donor[J]. Biofuels, 2018, 47(11):1-5. [8] BENDOVA D H, WEIDLICH D T. Application of diffusion dialysis in hydrometallurgical separation of nickel from spent Raney Ni catalyst[J]. Sep Sci Technol, 2017, 53(9):1-5. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=10.1080/01496395.2017.1329839 [9] LUO G H, WANG Y L, SUN D M, XU X, JIN H B. Effect of preparation process on compressive strength and hydrogenation performance of Raney-Ni/Al2O3 catalyst[J]. China Pet Process Petrochem Technol, 2017, 19(2):14-20. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgsyjgysyhgjs201702003 [10] LEI H, ZHEN S, TAN D L, BAO X H, MU X H, ZONG B N, MIN E Z. Preparation of novel raney-Ni catalysts and characterization by XRD, SEM and XPS[J]. Appl Catal A:Gen, 2001, 214(1):69-76. doi: 10.1016/S0926-860X(01)00481-1 [11] 雷浩.快凝Ni-Al合金结构及衍生骨架Ni催化剂加氢特性的研究[D].北京: 中国科学院研究生院, 2003. http://d.wanfangdata.com.cn/Thesis/Y578041LEI Hao. Study on hydrogenation characteristics of rapidly solidified Ni-Al alloy structure and derived framework Ni catalyst[D]. Beijing: Graduate School of Chinese Academy of Sciences, 2003. http://d.wanfangdata.com.cn/Thesis/Y578041 [12] 孙蛟, 任国卿, 黄玉辉, 陈晓蓉, 梅华.焙烧温度对CuMgAl催化剂催化糠醛气相加氢制糠醇性能的影响[J].燃料化学学报, 2017, 45(1):78-85. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18960.shtmlSUN Jiao, REN Guo-qing, HUANG Yu-hui, CHEN Xiao-rong, MEI Hua. Effect of calcination temperature on the catalytic performance of CuMgAl catalysts for furfural gas phase selective hydrogenation to furfuryl alcohol[J]. J Fuel Chem Technol, 2017, 45(1):78-85. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18960.shtml [13] YUAN P, LIU Z Y, SUN H J, LIU S C. Influence of calcination temperature on the performance of Cu-Al-Ba catalyst for hydrogenation of esters to alcohols[J]. Acta Phys Sin, 2010, 26(8):2235-2241. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=wlhxxb201008028 [14] 邓红, 韦藤幼, 童张法.超声浸渍法制备吗啉催化剂及其催化性能[J].化工进展, 2015, 34(2):425-446. http://d.old.wanfangdata.com.cn/Periodical/hgjz201502022DENG Hong, WEI Teng-you, TONG Zhang-fa. Preparation of catalyst for morpholine synthesis by ultrasonic irradiation impregnation method and its catalytic behaviors[J]. Chem Ind Eng Prog, 2015, 34(2):425-446. http://d.old.wanfangdata.com.cn/Periodical/hgjz201502022 [15] LIU Z X, WANG Y H, LI J R, ZHANG R G. The effect of γ-Al2O3 surface hydroxylation on the stability and nucleation of Ni in Ni/γ-A2O3 catalyst:A theoretical study[J]. Rsc Adv, 2014, 4(26):13280-13292. doi: 10.1039/c3ra46352d [16] LEI H, SONG Z, BAO X H, MU X H. XRD and XPS studies on the ultra-uniform Raney-Ni catalyst prepared from the melt-quenching alloy[J]. Surf Interface Anal, 2010, 32(1):210-213. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=ab2a48c69376aec649cb6234ceab6d42 [17] MEDGYES B. Electrochemical migration of Ni and ENIG surface finish during environmental test contaminated by NaCl[J]. J Mater Sci Mater Electron, 2017, 28(24):1-7. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=e1932810e5bd623c2abaf28936ba2a32 [18] WANG A L, YIN H B, LU H H, XUE J J, REN M, JIANG T S. Effect of organic modifiers on the structure of nickel nanoparticles and catalytic activity in the hydrogenation of p-nitrophenol to p-aminophenol[J]. ACS J Surfaces Colloid, 2009, 25(21):12736-12741. doi: 10.1021/la901815b [19] ATUL B, BRUNO T, PHILIPP R V R, ATSUSHI U. Impact of K and Ba promoters on CO2 hydrogenation over Cu/Al2O3 catalysts at high pressure[J]. Catal Sci Technol, 2013, 3(3):767-778. doi: 10.1039/C2CY20604H [20] BERND H, MATTHIAS H, LOUIS A C, RANDALL Q S. Characterization of acidic OH groups in zeolites of different types:An Interpretation of NH3-TPD results in the Light of confinement effects[J]. J Phys Chem, 2016, 106(15):21-36. http://www.researchgate.net/publication/231631196_Characterization_of_Acidic_OH_Groups_in_Zeolites_of_Different_Types_An_Interpretation_of_NH3-TPD_Results_in_the_Light_of_Confinement_Effects [21] BAGNASCO G, BENEˇS L, GALLI P, MASSUCCI M, PATRONO P, TURCO M, ZIMA V. TG/DTA, XRD and NH3-TPD characterization of layered VOPO4·2H2O and its Fe3+-substituted compound[J]. J Therm Anal Calor, 1998, 52(2):615-630. doi: 10.1023/A:1010136126445 -





 下载:
下载: