Preparation and evaluation of activated carbon-based desulfurization adsorbent by one-step method at low temperature
-
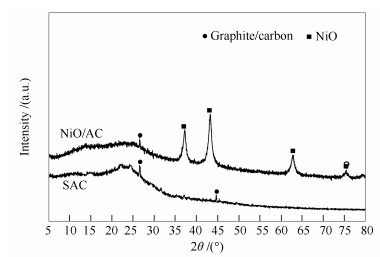
摘要: 以木屑为原料,在低温条件下一步法制得活性炭基吸附剂,考察了吸附剂制备条件和液-固、气-固吸附条件对吸附剂脱硫性能的影响。结果表明,吸附剂的最佳制备条件为,浸渍液与木屑质量比为1:1,浸渍液中硝酸质量分率为30%、吸附剂表面NiO负载量为5%,常温下浸渍24 h,400℃焙烧3 h。该吸附剂在0.2 g吸附剂/10 mL模拟油、温度为40℃及时间为5 h的液-固吸附脱硫的条件下,脱硫率为28.36%,吸附四次后饱和吸附硫容量可达2.34 mgS/g;在气-固吸附温度为250℃、空速为6.3 h-1的条件下,饱和吸附硫容量为2.37 mgS/g;高温气-固吸附脱硫对吸附剂的影响表明,与脱硫前相比,吸附剂在比表面积、总孔体积、微孔体积均有明显提高,这说明气-固吸附脱硫过程同时实现了活性炭的扩孔活化。甲苯溶剂再生实验表明,经五次再生后吸附剂的再生性能均可达90%以上。Abstract: Activated carbon was prepared by one-step method at low temperature using sawdust as material. Effect of preparation and adsorption conditions among liquid-solid and gas-solid on desulfurization performance was discussed. The results show that the optimum preparation conditions are as follows:mass ratio of 1:1 for impregnation liquid (with HNO3 mass fraction of 30%) and sawdust, NiO loading of 5% on surface of adsorbent, impregnation time of 24 h under room temperature and calcination time of 3 h under 400℃. Under adsorbent mass/DBT volume of 0.2 g/10 mL, liquid-solid adsorption temperature of 40℃ and adsorption time of 5 h, desulfurization efficiency can reach 28.36%. After continuous adsorption 4 times saturated adsorption sulfur capacity is up to 2.34 mgS/g. Under gas-solid adsorption temperature of 250℃ and adsorption velocity of 6.3 h-1, adsorption sulfur capacity can reach 2.37 mgS/g. The effect of high temperature gas-solid adsorption desulfurization on adsorbent indicates that BET specific surface area, total pore volume and micropore volume of adsorbent have been improved obviously, which show that the gas-solid adsorption desulfurization process has realized the reaming activation of activated carbon simultaneously. The regeneration experiment of toluene solvent shows that the regeneration performance can reach more than 90% after 5 times cycle.
-
Key words:
- adsorbent /
- preparation /
- dibenzothiophene /
- adsorption /
- desulfurization
-
表 1 波尔滴定法测定不同活性炭样品的表面含氧官能团
Table 1 Boehm titration oxygen-containing functional groups on the surface among different activated carbon samples
Sample Surface acidic functional group w/(mmol·g-1) carboxyl lactone phenolic hydroxyl total SAC 0.2902 0.2009 0.2324 0.7435 NiO/AC(0) 0.3413 0.2310 0.2517 0.8240 NiO/AC(10%) 0.4905 0.3009 0.3908 1.1822 NiO/AC(20%) 0.5712 0.3316 0.4349 1.3377 NiO/AC(30%) 0.6814 0.3508 0.4422 1.4744 NiO/AC(40%) 0.7508 0.3735 0.4628 1.5871 remarks: the data in brackets after NiO/AC in column samples represent the mass fraction of nitric acid in the impregnating solution 表 2 活性炭样品的孔结构特征
Table 2 Specific surface area and pore structure parameters of the activated carbon
Sample Surface area A/(m2·g-1) Micropore volume v/(cm3·g-1) Total pore volume v/(cm3·g-1) SAC 70.99 0.047 0.060 NiO/AC 48.42 0.045 0.062 ANiO/AC 106.89 0.078 0.098 -
[1] 沈俭一, 石国军.燃料油深度加氢脱硫催化剂的研究进展[J].石油化工, 2008, 37(11):1111-1120. doi: 10.3321/j.issn:1000-8144.2008.11.001SHEN Jian-yi, SHI Guo-jun. Research progress of catalysts for fuel deep hydrodesulfurization[J]. Petrochem Technol, 2008, 37(11):1111-1120 doi: 10.3321/j.issn:1000-8144.2008.11.001 [2] 李文秀, 崔安磊, 范俊刚, 孙向乐, 张志刚.载铜球形活性炭的制备及其吸附脱硫性能的研究[J].燃料化学学报, 2013, 41(5):613-618. http://www.ccspublishing.org.cn/article/id/100032910LI Wen-xiu, CUI An-lei, FAN Jun-gang, SUN Xiang-le, ZHANG Zhi-gang. Preparation and study on adsorption desulfurization performance over copper loaded spherical activated carbon[J]. J Fuel Chem Technol, 2013, 41(5):613-618. http://www.ccspublishing.org.cn/article/id/100032910 [3] 李秀萍, 赵荣祥, 苏勋建, 艾东.磷钨酸功能化氮化碳的制备及其氧化脱硫研究[J].燃料化学学报, 2015, 43(7):870-875. http://www.ccspublishing.org.cn/article/id/100033374LI Xiu-ping, ZHAO Rong-xiang, SU Xun-jian, AI Dong. Preparation and study on oxidation desulfurization over phosphotungstic acid functionalized carbon nitride[J]. J Fuel Chem Technol, 2015, 43(7):870-875. http://www.ccspublishing.org.cn/article/id/100033374 [4] 王建龙, 赵地顺, 周二鹏, 董芝.吡啶类离子液体在汽油萃取脱硫中的应用研究[J].燃料化学学报, 2007, 35(3):293-296. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rlhxxb200703008WANG Jian-long, ZHAO Di-shun, ZHOU Er-peng, DONG Zhi. Application of pyridinium ionic liquids in desulfurization of gasoline extraction[J]. J Fuel Chem Technol, 2007, 35(3):293-296. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rlhxxb200703008 [5] 唐晓东, 袁娇阳, 李晶晶, 张永汾, 胡涛.吡啶离子液体催化作用下的FCC汽油烷基化脱硫[J].燃料化学学报, 2015, 43(4):442-448. http://www.ccspublishing.org.cn/article/id/100033317TANG Xiao-dong, YUAN Jiao-yang, LI Jing-jing, ZHANG Yong-fen, HU Tao. Alkylation desulfurization of FCC gasoline catalyzed by pyridine ionic liquids[J]. J Fuel Chem Technol, 2015, 43(4):442-448. http://www.ccspublishing.org.cn/article/id/100033317 [6] 张存, 王峰, 潘小玉, 刘晓勤.酸性离子液体萃取-氧化模拟油品脱硫研究[J].燃料化学学报, 2011, 39(9):689-693. http://d.wanfangdata.com.cn/Periodical_rlhxxb201109009.aspxZHANG Cun, WANG Feng, PAN Xiao-yu, LIU Xiao-qin. Study on desulfurization performance for model fuel by acid ionic liquid extraction-oxidation[J]. J Fuel Chem Technol, 2011, 39(9):689-693. http://d.wanfangdata.com.cn/Periodical_rlhxxb201109009.aspx [7] 唐晓东, 李林, 曾雪玲, 赖先熔.汽油活性炭基脱硫吸附剂的制备与评价[J].燃料化学学报, 2009, 37(5):629-634. http://www.cnki.com.cn/Article/CJFDTOTAL-RLHX200905023.htmTANG Xiao-dong, LI Lin, ZEGN Xue-ling, LAI Xian-rong. Preparation and evaluation of gasoline adsorption desulfurization over activated carbon based adsorbent[J]. J Fuel Chem Technol, 2009, 37(5):629-634. http://www.cnki.com.cn/Article/CJFDTOTAL-RLHX200905023.htm [8] SALEH T A, DANMALIKI G I. Influence of acidic and basic treatments of activated carbon derived from waste rubber tires on adsorptive desulfurization of thiophenes[J]. J Taiwan Inst Chem E, 2016, 60:460-468. doi: 10.1016/j.jtice.2015.11.008 [9] MENG X, HUANG H, WENG H, SHI L. Ni/ZnO-based adsorbents supported on Al2O3, SiO2, TiO2, ZrO2:A comparison for desulfurization of model gasoline by reactive adsorption[J]. Bull korean Chem Soc, 2012, 33(10):3213-3217. doi: 10.5012/bkcs.2012.33.10.3213 [10] SENTORUN-SHALABY C, SAHA SK, MA X, SONG C. Mesoporous-molecular-sieve-supported nickel sorbents for adsorptive desulfurization of commercial ultra-low-sulfur diesel fuel[J]. Appl Catal B:Environ, 2011, 101(3/4):718-726. http://canli.dicp.ac.cn/Gruop Seminars Pdf/20110327ylzhang.pdf [11] KHAN N A, JHUNG S H. Low-temperature loading of Cu+ species over porous metal-organic frameworks (MOFs) and adsorptive desulfurization with Cu+-loaded MOFs[J]. J Hazard Mater, 2012, 237/238(17):180-5. http://www.doc88.com/p-9989446071241.html [12] FAŁTYNOWICZ H, KACZMARCZYK J, KUŁA Ż Y ŃSKI M. Preparation and characterization of activated carbons from biomass material-giant knotweed (Reynoutria sachalinensis)[J]. Open Chem, 2015, 13(1):1150-1156. http://cn.bing.com/academic/profile?id=62208c87c78a8da5f36913061f6a0fc7&encoded=0&v=paper_preview&mkt=zh-cn [13] CAO B, SHEN W, LIU Y. Adsorption desulphurization of gasoline by silver loaded onto modified activated carbons[J]. Adsorption Technology, 2008, 26(4):225-231. doi: 10.1260/026361708786934451 [14] 查庆芳, 高南星, 李兆丰, 卓海波, 张玉贞.石油焦系活性炭的吸附脱硫[J].燃料化学学报, 2007, 35(2):192-197. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rlhxxb200702012ZHA Qing-fang, GAO Nan-xing, LI Zhao-feng, ZHUO Hai-bo, ZHANG Yu-zhen. Adsorption desulfurization by activated carbon from petroleum coke[J]. J Fuel Chem Technol, 2007, 35(2):192-197. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rlhxxb200702012 [15] 程凯鹏. CeO2/AC和Ag/AC燃油脱硫吸附剂的制备及性能研究[D]. 开封: 河南大学, 2016.CHENG Kai-peng. Study on preparation and performance for adsorption desulfurization over CeO2/AC and Ag/AC adsorbents[D]. Kaifeng: Henan University, 2016. [16] LEI M, TONG S T, YU W. Discussion on the Boehm titration method used in analysis of surface oxygen functional groups on activated carbon[J]. Carbon Techniques, 2011, 30(2):17-19. http://www.sid.ir/En/Journal/Reference.aspx?ID=434725 [17] 何小超, 郑经堂, 于维钊.燃油活性炭吸附深度脱硫的机理及研究进展[J].炼油技术与工程, 2007, 37(7):8-12. http://d.old.wanfangdata.com.cn/Periodical/lysj200707003HE Xiao-chao, ZHENG Jing-tang, YU Wei-zhao. Mechanism and research progress of deep adsorption desulfurization over fuel activated carbon[J]. Pet Ref Eng, 2007, 37(7):8-12. http://d.old.wanfangdata.com.cn/Periodical/lysj200707003 [18] SHEN W, LI Z, LIU Y. Surface chemical functional groups modification of porous carbon[J]. Recent Patents on Chemical Engineering, 2008, 1(1):27-40. doi: 10.2174/2211334710801010027 [19] 米尼奥维奇.硝酸盐岩石快速全分析[M].北京:科学出版社, 1956.Miniovich. Rapid Total Analysis of Nitrate Rock[M]. Beijing:Science Press, 1956. [20] 张微, 葛庆杰, 徐恒泳.镍前驱体对非负载型镍催化剂上甲烷分解活性的影响[J].催化学报, 2010, 31(11):1358-1362. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=cuihuaxb201011009ZHANG Hui, GE Qing-jie, XU Heng-yong. Effect of precursors on methane decomposition activity on non-loaded nickel catalysts[J]. J Catal, 2010, 31(11):1358-1362. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=cuihuaxb201011009 [21] 王振永. 活性炭基脱硫剂FCC汽油吸附脱硫性能评价及物化性质表征[D]. 青岛: 中国海洋大学, 2007.WANG Zhen-yong. Adsorption desulfurization process of FCC gasoline and characterization physicochemical properties for activated carbon adsorbents[D]. Qingdao: Ocean University of China, 2007. [22] 余谟鑫, 李忠, 夏启斌, 王书文.表面负载不同金属离子的活性炭吸附二苯并噻吩[J].功能材料, 2006, 37(11):1816-1818. doi: 10.3321/j.issn:1001-9731.2006.11.039YU Mo-xin, LI Zhong, XIA Qi-bin, WANG Shu-wen. Adsorption desulfurization for dibenzothiophene over active carbon based adsorbents with different metal ions[J]. Functional materials, 2006, 37(11):1816-1818. doi: 10.3321/j.issn:1001-9731.2006.11.039 -





 下载:
下载: