Loading strategy for the active components of monolithic catalyst and its influences on the microwave enhanced catalytic combustion of toluene
-
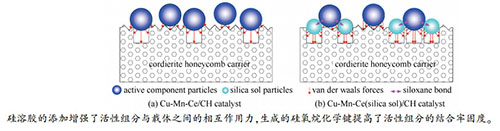
摘要: 针对催化剂活性组分脱落问题,采用载体预处理和添加硅溶胶的策略来强化活性组分负载,微波单模腔中催化燃烧甲苯以考察催化剂活性,并对牢固负载的催化剂进行表征分析。研究表明,常温下采用10%盐酸溶液对蜂窝状堇青石(CH)载体预处理、硅溶胶添加量与载体吸水量比值为0.125条件下所制备的Cu-Mn-Ce(硅溶胶)/CH催化剂脱落率为0.0129%,明显低于Cu-Mn-Ce/CH催化剂的0.950%。Cu-Mn-Ce(硅溶胶)/CH催化剂具有更小的活性颗粒尺寸、更大的比表面积和更多样的活性晶体,在甲苯进气浓度1000 mg/m3、进气量0.12 m3/h、微波功率200 W和床层温度350℃条件下,催化剂对甲苯的催化燃烧效率和矿化率分别为98.5%和87.9%;连续实验43 h后,催化剂活性保持稳定且活性组分脱落率低(0.0328%)。硅溶胶的添加增强了活性组分与载体之间的相互作用力,生成的硅氧烷化学键提高了活性组分的结合牢固度。Abstract: Aiming at solving the shedding problem of active components, a loading strategy that includes carrier pretreatment and addition of silica sol was adopted to strengthen the combination of the active components with the carrier. Catalytic activity of the catalyst was investigated in toluene combustion by using microwave single-mode cavity, and high-firmness catalysts were characterized subsequently. The study showed that the shedding rate of Cu-Mn-Ce(silica sol)/cordierite honeycomb(CH) catalyst prepared under conditions of 10% hydrochloric acid pretreatment at room temperature and 0.125 of the mass ratio of silica sol to water absorption amount of CH carrier was 0.0129%, which was much lower than 0.950% of Cu-Mn-Ce/CH catalyst. Cu-Mn-Ce(silica sol)/CH catalyst had smaller active particles, larger specific surface area and more active crystals than Cu-Mn-Ce/CH catalyst. Under conditions of 1000 mg/m3 of initial concentration, 0.12 m3/h of air flow, 200 W of microwave power and 350 ℃ of bed temperature, the removal and mineralization rates of toluene by Cu-Mn-Ce(silica sol)/CH catalyst were 98.5% and 87.9%, respectively. The Cu-Mn-Ce(silica sol)/CH catalyst owned high catalytic activity and stability after 43 h run, and the shedding rate of active components was 0.0328%. The addition of silica sol could enhance the interaction forces between the active components and the catalyst carrier, and the formation of siloxane chemical bonds could greatly improve the connection of active components to prolong the service life of the catalyst.
-
Key words:
- firmness /
- silica sol /
- microwave catalysis /
- toluene /
- mineralization /
- characterization
-
图 1 实验装置流程示意图
Figure 1 Schematic of the experimental device flow
1: air compressor; 2: silica gel column; 3: activated carbon column; 4: flowmeter; 5: syringe pump; 6: evaporation flask; 7: explosion-proof zeolite; 8: electric furnace; 9: buffer bottle; 10: inlet sampling point; 11: gas chromatograph; 12: microwave device; 13: water-cooling system; 14: K-type thermocouple; 15: fixed bed reactor; 16: outlet sampling point; 17: organic solvent bottle; 18: alkaline solution bottle
图 2 预处理后催化剂活性组分负载量和脱落率
Figure 2 Load and shedding rates of the active components of catalysts after pretreatment
G1: control group; G2: ultrasonic vibration; G3: 5%HNO3; G4: 10% HNO3; G5: 15% HNO3; G6: 30% HNO3; G7: 20%H2C2O4; G8: 30% H2C2O4; G9: 40% H2C2O4; G10: 27%NH3·H2O; G11: 10%HCl; G12: 0.5 mol/L NaOH
图 3 添加硅溶胶后催化剂活性组分负载量和脱落率
Figure 3 Load and shedding rates of the active components of catalysts after adding silica sol
k1=0; k2=0.0500; k3=0.0750; k4=0.1000; k5=0.1063; k6=0.1125; k7=0.1188; k8=0.1250; k9=0.1500; msilica sol/mwater absorption refer to the mass ratio of silica sol to water absorption amount of the carrier
图 8 床层温度300 ℃下两种催化剂对甲苯的催化降解
Figure 8 Catalytic degradation of toluene at 300 ℃ of bed temperature over two catalysts separately
■: Cu-Mn-Ce/CH catalyst; ●: Cu-Mn-Ce(silica sol)/CH catalyst reaction conditions: toluene initial concentration 1000 mg/m3, air flow rate 0.12 m3/h, catalyst volume 6.15×10-5 m3, bed height 100 mm, bed temperature 300 ℃, Cu-Mn-Ce/CH catalyst:Pheating=100 W, Pinsulation=75 W; Cu-Mn-Ce (silica sol)/CH catalyst: Pheating=250 W, Pinsulation=150 W
图 9 不同床层温度下Cu-Mn-Ce(硅溶胶)/CH催化剂对甲苯的催化降解
Figure 9 Catalytic degradation of toluene at different bed temperatures over Cu-Mn-Ce(silica sol)/CH catalyst
■: 150 ℃(Pheating=100 W, Pinsulation=75 W); □: 200 ℃(Pheating=100 W, Pinsulation=87 W); ▲: 250 ℃(Pheating=200 W, Pinsulation=105 W); △: 300 ℃(Pheating=250 W, Pinsulation=150 W); ●: 350 ℃(Pheating=250 W, Pinsulation=220 W); ○: 400 ℃(Pheating=300 W, Pinsulation=275 W)
图 10 两种催化剂对甲苯的矿化率
Figure 10 Mineralization rate of toluene over two different catalysts
■: mineralization rate of toluene over the Cu-Mn-Ce(silica sol)/CH catalyst; □: mineralization rate of toluene over the Cu-Mn-Ce/CH catalyst; ●: Cu-Mn-Ce(silica sol)/CH catalyst activity; ○: Cu-Mn-Ce/CH catalyst activity reaction conditions: toluene concentration 1000 mg/m3, air flow rate 0.12 m3/h, catalystvolume 6.15×10-5 m3, bed height 100 mm, bed temperature 350 ℃, Pheating=250 W, Pinsulation=220 W
图 11 催化剂的稳定性测试
Figure 11 Stability test of catalysts
■: toluene removal rate; ●: active components shedding rate reaction conditions: toluene concentration 1000 mg/m3, air flow rate 0.12 m3/h, catalyst volume 6.15×10-5 m3, bed height 100 mm, temperature 350 ℃, Cu-Mn-Ce/CH catalyst: Pheating=150 W, Pinsulation=123 W; Cu-Mn-Ce(silica sol)/CH catalyst: Pheating=250 W, Pinsulation=220 W
表 1 预处理后堇青石载体的吸水率
Table 1 Water absorption of the cordierite carrier after pretreatment
Pretreatmentsolution Controlgroup Ultrasonic vibration 5%HNO3 10%HNO3 15%HNO3 30%HNO3 20%H2C2O4 30%H2C2O4 40%H2C2O4 27%NH3·H2O 10%HCl 0.5 mol/LNaOH Water absorption /% 38.26 44.62 44.16 56.63 60.07 43.04 67.91 59.66 51.00 50.53 53.18 52.19 note:5%, 10%, 40%, 27%, etc. are the mass percentages when the acid and alkali solutions are diluted 表 2 催化剂的比表面积和孔结构参数
Table 2 Specific surface area and pore structure parameters of different carriers and catalysts
Sample BET special surface area A/(m2·g-1) Micropore area A/(m2·g-1) Total pore volume v/ (cm3·g-1) Micropore volume v/(cm3·g-1) Average pore size d/nm CH carrier 0.98 0.47 0.00080 0.00050 9.56 CH carrier(acid etching) 2.80 0.37 0.0036 0.00024 20.18 New/Cu-Mn-Ce/CH catalyst 13.93 8.47 0.052 0.0065 32.90 New/Cu-Mn-Ce(silica sol)/CH catalyst 28.28 12.72 0.089 0.0098 22.99 Old/Cu-Mn-Ce/CH catalyst 9.04 3.27 0.016 0.0017 26.38 Old/Cu-Mn-Ce(silica sol)/CH catalyst 19.24 10.70 0.074 0.0035 18.23 表 3 实验前后两种催化剂的活性组分牢固度分析
Table 3 Firmness analysis of the active components of two catalysts before and after stability test
Sample Quality of cordierite carrier after pretreatment m0/g Quality of supported catalyst m1/g Initial active components loading w1/% Quality of catalyst after firmness test m2/g Active components shedding rate △w/% Final active components loading w2/% New/Cu-Mn-Ce/CH catalyst 31.8772 34.8520 9.33 34.8105 0.119 9.20 New/Cu-Mn-Ce (silica sol)/CH catalyst 32.4818 35.8107 10.25 35.8048 0.0165 10.23 Old/Cu-Mn-Ce/CH catalyst 33.2763 36.3777 9.32 36.2455 0.363 8.92 Old/Cu-Mn-Ce (silica sol)/CH catalyst 32.3773 35.7025 10.27 35.6908 0.0328 10.23 -
[1] ZHANG X M, XUE Z G, LI H, YAN L, YANG Y, WANG Y, DUAN J C, LI L, CHAI F H, CHENG M M, ZHANG W Q. Ambient volatile organic compounds pollution in China[J]. J Environ Sci, 2017, 55(5):69-75. https://www.sciencedirect.com/science/article/abs/pii/S1001074216302662 [2] CHEN K Y, ZHU L Z, YANG K. Tricrystalline TiO2 with enhanced photocatalytic activity and durability for removing volatile organic compounds from indoor air[J]. J Environ Sci, 2015, 32(6):189-195. http://www.ncbi.nlm.nih.gov/pubmed/26040745 [3] BARI M A, KINDZIERSKI W B. Ambient volatile organic compounds (VOCs) in Calgary, Alberta:Sources and screening health risk assessment[J]. Sci Total Environ, 2018, 631-632(8):627-640. http://smartsearch.nstl.gov.cn/paper_detail.html?id=495656851c8078bf22d3f6dd1c0c5b0e [4] DUMANOGLU Y, KARA M, ALTIOK H, ODABASI M, ELBIR T, BAYRAM A. Spatial and seasonal variation and source apportionment of volatile organic compounds (VOCs) in a heavily industrialized region[J]. Atmos Environ, 2014, 98(12):168-178. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=a66f11f49bbb54ec31bd3d9cfb105cdf [5] 闻择.国家颁布"十三五" VOCs污染防治方案严限重点地区石油化工等高排放项目[J].上海化工, 2017, 42(11):3. http://www.cqvip.com/main/zcps.aspx?c=1&id=83727271504849554949484852WEN Ze. The"13th Five-Year-Plan"for pollution prevention and control was issued by Chinese government to limit high-emission projects such as petrochemical industry in heavily polluted areas[J]. Shanghai Chem Ind, 2017, 42(11):3. http://www.cqvip.com/main/zcps.aspx?c=1&id=83727271504849554949484852 [6] 施旭荣.上海市宝山区挥发性有机化合物排放治理效果评价与对策研究[D].乌鲁木齐: 新疆大学, 2019. http://cdmd.cnki.com.cn/Article/CDMD-10755-1019609747.htmSHI Xu-rong. Effect evaluation and countermeasure study on volatile organic compounds emission control in Baoshan district, shanghai[D]. Urumchi: Xinjiang University, 2019. http://cdmd.cnki.com.cn/Article/CDMD-10755-1019609747.htm [7] 黄青斌.挥发性有机物催化氧化技术及催化剂研究进展[J].化学试剂, 2019, 41(2):107-112. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=huaxsj201902001HUANG Qing-bin. Research progress in catalytic oxidation technology and catalysts for volatile organic compounds[J]. Chem Reagents, 2019, 41(2):107-112. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=huaxsj201902001 [8] 卜龙利, 刘海楠, 王晓晖, 张浩, 孙剑宇, 杨力.不同加热方式下催化氧化甲苯的性能研究[J].环境化学, 2013, 32(8):1524-1531. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hjhx201308017BO Long-li, LIU Hai-nan, WANG Xiao-hui, ZHANG Hao, SUN Jian-yu, YANG Li. Study on the catalytic oxidation of toluene under different heating modes[J]. Environ Chem, 2013, 32(8):1524-1531. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hjhx201308017 [9] 张钰彩, 卜龙利, 王晓晖, 刘海楠, 张浩.微波加热下苯的催化氧化性能研究[J].环境科学, 2012, 33(8):2759-2765. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hjkx201208035ZHANG Yu-cai, BO Long-li, WANG Xiao-hui, LIU Hai-nan, ZHANG Hao. Study on catalytic oxidation of benzene by microwave heating[J]. Environ Sci, 2012, 33(8):2759-2765. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hjkx201208035 [10] 卜龙利, 杨力, 孙剑宇, 梁欣欣, 虎雪姣, 孟海龙.双组分VOCs的催化氧化及动力学分析[J].环境科学, 2014, 35(9):3302-3308. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hjkx201409010BO Long-li, YANG Li, SUN Jian-yu, LIANG Xin-xin, HU Xue-jiao, MENG Hai-long. Catalytic oxidation of two-component VOCs and kinetic analysis[J]. Environ Sci, 2014, 35(9):3302-3308. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hjkx201409010 [11] 刘海楠.二氧化钛复合型催化剂制备及其微波辅助催化氧化甲苯性能试验研究[D].西安: 西安建筑科技大学, 2013.LIU Hai-nan. Preparation of titania composite catalyst and its performance in microwave-assisted catalytic oxidation of toluene[D]. Xi'an: Xi'an University of Architecture and Technology, 2013. [12] 廖建波.挥发性有机物微波辅助催化氧化性能试验研究[D].西安: 西安建筑科技大学, 2011. http://d.wanfangdata.com.cn/thesis/D243709LIAO Jian-bo. Study on the performance of microwave-assisted catalytic oxidation of volatile organic compound[D]. Xi'an: Xi'an University of Architecture and Technology, 2011. http://d.wanfangdata.com.cn/thesis/D243709 [13] 史志铭, 梁开明, 顾守仁.元素掺杂对堇青石晶体结构及热膨胀系数的作用[J].现代技术陶瓷, 2000, 21(2):18-23. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QK200001039360SHI Zhi-ming, LIANG Kai-ming, GU Shou-ren. Effects of elements-doping on the crystal structure and thermal expansion coefficient of cordierite[J]. Adv Ceram, 2000, 21(2):18-23. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QK200001039360 [14] ZHAI Y Q, XIONG J M, LI C Q, XU X, LUO G H. Influence of preparation method on performance of a metal supported perovskite catalyst for combustion of methane[J]. J Rare Earth, 2010, 28(1):54-58. doi: 10.1016/S1002-0721(09)60050-8 [15] 陆彦彬, 马彪, 张伟辰. HZSM-5/整体式催化剂的制备及其在甲醇制汽油反应中的应用[J].辽宁石油化工大学学报, 2015, 35(1):12-15. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=fssyxyxb201501004LU Yan-bin, MA Biao, ZHANG Wei-chen. Preparation of HZSM-5/monolithic catalyst and its application in conversion of methanol to gasoline[J]. J Liaoning Shihua Univ, 2015, 35(1):12-15. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=fssyxyxb201501004 [16] 陶明. TS-1/堇青石整体催化剂的制备及其催化苯酚羟基化的性能研究[D].杭州: 浙江工业大学, 2016. http://cdmd.cnki.com.cn/Article/CDMD-10337-1017027496.htmTAO Ming. Synthesis of TS-1/cordierite monolith catalyst and its catalytic performance of phenol hydroxylation[D]. Hangzhou: Zhejiang University of Technology, 2016. http://cdmd.cnki.com.cn/Article/CDMD-10337-1017027496.htm [17] 黄海凤, 周小燕, 卢晗锋, 俞河, 陈银飞.原位沉淀技术制备整体型Mn/Ti-Si/堇青石选择性催化还原催化剂[J].中国电机工程学报, 2011, 31(17):50-54. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgdjgcxb201117008HUANG Hai-feng, ZHOU Xiao-yan, LU Han-feng, YU He, CHEN Yin-fei. Monolithic Mn/Ti-Si/cordierite catalyst prepared by In-situ deposition for SCR-DeNOx[J]. Proc CSEE, 2011, 31(17):50-54. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgdjgcxb201117008 [18] 宋万仓.金属基不锈钢表面上Al2O3涂层的制备[D].辽宁: 大连理工大学, 2010.SONG Wan-cang. Preparation of Al2O3 coating on stainless steel substrate[D]. Liaoning: Dalian University of Technology, 2010. [19] 陈春波, 李平, 隋志军, 王寅, 朱连利, 赫崇衡.大比表面积高牢固度堇青石蜂窝涂层的制备[J].工业催化, 2010, 18(3):40-45. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=gych201003007CHEN Chun-bo, LI Ping, SUI Zhi-jun, WANG Yin, ZHU Lian-li, HE Chong-heng. Preparation of cordierite honeycomb coating with large surface area and high adhesive capacity[J]. Ind Catal, 2010, 18(3):40-45. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=gych201003007 [20] 贺振富, 邵潜, 沈宁元.载体预处理对催化剂涂层结构的影响[J].石油学报(石油加工), 2001, 17(3):83-85. http://www.cnki.com.cn/Article/CJFDTotal-SXJG200103014.htmHE Zhen-fu, SHAO Qian, SHEN Ning-yuan. Influence of pretreatment on structure of catalytic washcoat[J]. Acta Pet Sin(Pet Proc Sect), 2001, 17(3):83-85. http://www.cnki.com.cn/Article/CJFDTotal-SXJG200103014.htm [21] 刘海楠, 卜龙利, 王晓晖, 张浩, 孙剑宇, 杨力, 蔡力栋.二氧化钛复合型催化剂制备及其微波辅助催化氧化甲苯性能[J].环境科学学报, 2013, 33(6):1720-1727. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hjkxxb201306027LIU Hai-nan, BO Long-li, WANG Xiao-hui, ZHANG Hao, SUN Jian-yu, YANG Li, CAI Li-dong. Preparation of titania composite catalyst and its performance in microwave-assisted catalytic oxidation of toluene[J]. Acta Sci Circum, 2013, 33(6):1720-1727. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hjkxxb201306027 [22] 李凯.蜂窝状Mn-Ce/TiO2/CC脱硝催化剂的涂覆技术及性能研究[D].太原: 太原理工大学, 2013. http://cdmd.cnki.com.cn/Article/CDMD-10112-1013355319.htmLI Kai. Coating technology and the performance of Mn-Ce/TiO2/CC honeycomb catalysts for NO removal[D]. Taiyuan: Taiyuan University of Technology, 2013. http://cdmd.cnki.com.cn/Article/CDMD-10112-1013355319.htm [23] BO L L, SUN S Y. Microwave-assisted catalytic oxidation of gaseous toluene with a Cu-Mn-Ce/cordierite honeycomb catalyst[J]. Front Chem Sci Eng, 2019, 13(2):385-392. http://www.cqvip.com/QK/71238X/20192/7002258221.html [24] SALKER A V, WEISWEILER W. Catalytic behaviour of metal based ZSM-5 catalysts for NOx reduction with NH3 in dry and humid conditions[J]. Appl Catal A:Gen, 2000, 203(2):221-229. doi: 10.1016/S0926-860X(00)00489-0 [25] 贺利娜, 卜龙利, 都琳, 宁轲, 刘嘉栋.微波催化燃烧气态甲苯特性及床层温度分布[J].中国环境科学, 2019, 39(8):3242-3248. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zghjkx201908014HE Li-na, BO Long-li, DU Lin, NING Ke, LIU Jia-dong. Microwave catalytic combustion of gaseous toluene and distribution of bed temperature[J]. China Environ Sci, 2019, 39(8):3242-3248. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zghjkx201908014 [26] 董亮.磷化物整体式催化剂的制备与加氢脱硫性能研究[D].北京: 北京化工大学, 2009. http://cdmd.cnki.com.cn/Article/CDMD-10010-2009261156.htmDONG Liang. Study on preparation and hydrodesulfurization properties of phosphide monolithic catalysts[D]. Beijing: Beijing University of Chemical Technology, 2009. http://cdmd.cnki.com.cn/Article/CDMD-10010-2009261156.htm [27] 马文娇, 黄琼, 陈英文, 祝社民, 沈树宝.堇青石负载Fe-Mn-O混合氧化物催化氧化甲苯的性能研究[J].现代化工, 2012, 32(3):26-29. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=xdhg201203007MA Wen-jiao, HUANG Qiong, CHEN Ying-wen, ZHU She-min, SHEN Shu-bao. Catalytic performance of Fe-Mn-O mixed oxides supported on cordierite for toluene catalytic combustion[J]. Mod Chem Ind, 2012, 32(3):26-29. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=xdhg201203007 [28] 张峰. SCR脱硝催化剂的抗硫再生性能和整体化制备研究[D].杭州: 浙江工业大学, 2010. http://d.wanfangdata.com.cn/thesis/Y1776177ZHANG Feng. Study on the performance of sulfur tolerance and regeneration and preparation of the monolithic catalyst for SCR denitration[D]. Hangzhou: Zhejiang University of Technology, 2010. http://d.wanfangdata.com.cn/thesis/Y1776177 [29] 薛红丹.中温原位催化还原NOx催化剂的制备及性能研究[D].哈尔滨: 哈尔滨工程大学, 2006. http://d.wanfangdata.com.cn/thesis/Y936352XUE Hong-dan. Study on preparation and properties of catalysts used for selective catalytic reduction of NOx[D]. Harbin: Harbin Engineering University, 2006. http://d.wanfangdata.com.cn/thesis/Y936352 [30] 孟海龙, 卜龙利, 刘嘉栋, 高波, 冯奇奇, 谭娜, 谢帅. CoCuMnOx光催化氧化多组分VOCs特性及其动力学[J].环境科学, 2016, 37(5):1670-1676. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hjkx201605010MENG Hai-long, BO Long-li, LIU Jia-dong, GAO Bo, FENG Qi-qi, TAN Na, XIE Shuai. CoCuMnOx Photocatalyzed oxidation of multi-component VOCs and kinetic analysis[J]. Environ Sci, 2016, 37(5):1670-1676. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hjkx201605010 [31] WANG D, ZAHNG L, LI J H, KAMASAMUDRAM K, EPLING W. NH3-SCR over Cu/SAPO-34-Zeolite acidity and Cu structure changes as a function of Cu loading[J]. Catal Today, 2014, 231(1):64-74. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=6f80b46c274d44628451d83a54d08ece [32] HU Y T, LV Z H, WEI C, YU S C, LIU M H, GAO C J. Separation and antifouling properties of hydrolyzed PAN hybrid membranes prepared via in-situ sol-gel SiO2 nanoparticles growth[J]. J Membrane Sci, 2018, 545(1):250-258. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=fb07f78ca4755757aff733d12c4c364b [33] 杨力, 卜龙利, 孙剑宇, 梁欣欣, 虎雪姣, 孟海龙.双组分甲苯、氯苯的微波辅助催化氧化及机理[J].环境工程学报, 2014, 8(11):4871-4879. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hjwrzljsysb201411050YANGA Li, BO Long-li, SUN Jian-yu, LIANG Xin-xin, HU Xue-jiao, MENG Hai-long. Microwave assisted catalytic oxidation of two-component toluene, chlorobenzene and relative removal mechanism[J]. Chin J Environ Eng, 2014, 8(11):4871-4879. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hjwrzljsysb201411050 [34] WU D F, ZHANG Y H, LI Y D. Mechanical stability of monolithic catalysts:scattering of washcoat connection and failure mechanism of active material[J]. Ind Eng Chem Res, 2013, 52(41):14713-14721. doi: 10.1021/ie402546q [35] Krijn P. de Jong. Synthesis of Solid Catalysts[M]. Weinheim: WILEY-VCH Verlag GmbH & Co. KGaA, 2009. [36] 周铭.涂膜附着机理与附着力促进剂的选择[J].现代涂料与涂装, 2007, 10(8):1-3. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=xdtlytz200708001ZHOU Ming. Connection mechanism of the coatings and selection of the connection accelerants[J]. Mod Paint Finish, 2007, 10(8):1-3. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=xdtlytz200708001 [37] ZAMARO J M, ULLA M A, MIRÓ E E. Zeolite washcoating onto cordierite honeycomb reactors for environmental applications[J]. Chem Eng J, 2005, 106(1):25-33. http://www.sciencedirect.com/science/article/pii/S1385894704003584 [38] KONG D Y, YANG H, WEI S, LI D Y, WANG J B. Gel-casting without de-airing process using silica sol as a binder[J]. Ceram Int, 2007, 33(2):133-139. doi: 10.1016/j.ceramint.2005.08.006 [39] 晋斌.硅溶胶及其在消防技术中的应用[J].消防科学与技术, 1989, (2):35-36. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QK000005636629JIN Bin. Silica sol and its application in fire protection technology[J]. Fire Sci Technol, 1989, (2):35-36. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QK000005636629 -





 下载:
下载: