Influence of oxygen on nitrogen distribution and transformation during straw pyrolysis
-
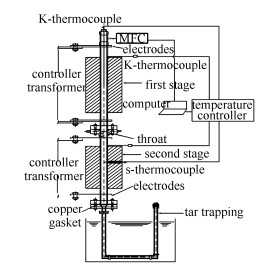
摘要: 以秸秆为原料,在两段式固定床反应器上模拟层燃工况进行热解实验,研究了氧气对热解过程中燃料氮迁移与转化的影响。通过对焦油中含氮化合物种类与含量的GC-MS分析,提出了热解过程中燃料氮转化的反应路径,并分析了氧气的影响。与惰性气氛下相比,氧气的引入降低了焦油与焦炭的产率,从而降低了焦油与焦炭中氮的分配比,增加了气体组分中氮的分配比。以蛋白质和氨基酸作为燃料氮的禀赋形态,其在热解过程中发生一系列一次反应,生成酰胺、胺类等初级焦油产物。初级焦油发生二次反应,进一步生成腈类及含氮杂环化合物等二级焦油产物。有氧条件下,焦油中酰胺、胺类等初级焦油成分的含量显著降低,腈类及含氮杂环化合物等二级焦油成分的含量升高。Abstract: To study influence of oxygen on distribution and transformation of fuel-N, pyrolysis experiments of straw were conducted in a two stage fixed bed reactor simulating the run conditions of grate firing. The conversion pathway of fuel-N was depicted by studying types and content of N-containing compounds in tar by GC-MS, and effect of oxygen was analyzed. Compared with inert atmosphere, tar and char yields decrease with introduction of oxygen, which leads to a decrease of N-distribution in tar and char and increase in gas fractions. Protein and amino acids are the main N-containing components in fuel, which initially go through a series of primary reactions, producing primary tar components like amides and amines. Then the primary tar goes through secondary reactions to yield secondary tar components including nitriles and N-heterocyclic compounds. In the presence of oxygen, content of primary tar components like amides and amines decrease significantly, and secondary tar components like nitriles and N-heterocyclic compounds increase.
-
Key words:
- straw /
- oxidative pyrolysis /
- N-distribution /
- conversion pathway
-
表 1 秸秆原料的工业分析及元素分析
Table 1 Proximate and ultimate analysis of straw sample
Proximate analysis wad/% Ultimate analysis wad/% M V A FC C H N S O 10.23 59.05 16.29 14.43 37.22 5.43 1.42 0.25 39.40 表 2 焦炭与焦油的产率
Table 2 Char and tar yields
φ(O2)/% 0* 0 0.5 1 5 Char w/% 35.20 35.05 34.5 33.64 28.17 Tar w/% 20.14 7.05 7.04 6.42 4.83 表 3 焦炭与焦油的元素分析
Table 3 Ultimate analysis of char and tar samples
φ(O2)/% 0* 0 0.5 1 5 tar char tar char tar char tar char tar char C 45.33 42.66 43.59 42.09 45.77 43.60 44.2 43.3 42.02 34.48 Ultimate H 7.98 2.10 8.49 2.37 8.16 2.19 8.23 2.11 8.34 1.55 analysis w/% S 0.42 0.37 0.46 0.36 0.51 0.40 0.37 0.36 0.38 0.39 N 1.49 1.67 1.42 1.68 1.79 1.66 1.40 1.65 0.88 1.31 表 4 三种工况下焦油中含氮化合物及其相对含量
Table 4 Nitrogen compounds and their relative concentrations from three working conditions
φ(O2)/% 0* 0 5.0 N-containing compounds relative content/% N-containing compounds relative content/% N-containing compounds relative content/% Amides methylurea 3.46 undec-10-ynoic acid (furan-2-ylmethyl)-amide 1.90 hydroxyproline anilide 4.28 N-(cyanomethyl)-foramide 2.26 2-propenamide 3.88 hydantoin 12.32 ethyl allophanate 4.74 carbamic acid, phenyl ester 2.60 2, 5-piperazine-dione 1.43 pyrazinamide 6.67 Amides 2-amino-oxazole 2.67 N-ethyl-2-propen-1-amine 4.28 aniline 6.42 1, 3-benzene-diamine 1.21 2-amino-4-methyl-5-hexynoic acid 1.80 2-methyl-2-propene-nitrile 3.46 cyclopropanecarbox-amine 3.78 6-amino-2-(4-aminophenyl) benzoxazole 1.44 3-amino-piperidin-2-one 4.06 2-amino-oxazole 1.17 N-benzyl-2-phenethylamine 4.06 Nitriles 3-amino-3-cyano-acrylic acid, methyl ester 1.01 2-pyrrodine-carbonitrile, 5-oxo- 3.80 3-dimethyl-amino-acrylonitrile 6.73 benzenepropane-nitrile, β-oxo- 4.91 3-amino-acrylonitrile 4.78 2-methyl-2-propenenitrile 1.80 cyanamide, 2-propenyl- 0.55 N-heterocyclic 3-methylpyridazine 5.12 1H-pyrazole, 1, 3-dimethyl- 0.12 1, 3-diazine 1.87 compounds 1, 3-diazine 4.26 3-methylpyridazine 4.77 H-pyrrole, 1-methyl- 6.90 1-methyl-Pyrrole 5.05 1, 3-diazine 1.99 pyridine 26.03 pyridine 3.17 pyridine 22.53 pyrrole 2.93 pyrrole 17.47 pyrrole 15.82 3-methyl-pyridine 2.70 4-pyridinol 6.78 4-methylpyridine 3.71 2-pyrimidin-amine 0.27 4-methylpyridine 4.97 2-methylpyrrolidine 2.08 piperazine 0.17 1, 5-dimethyl-pyzazole 4.01 2, 6-dimethyl-pyridine 2.07 4-ethenyl-pyrodine 0.76 5-methy-2-phenyl-indole 0.65 2-ethylpyridine 1.75 3, 5-dimethyl-pyridine 21.23 3, 6-Bis (N, N-dimethylamino)-9-methylcarbazole 1.77 4-pyridine-carboxyaldehyde 0.02 2-ethyl-acridine 0.98 2, 5-dimethylpyridine 2.01 2-ethenylpyridine 0.09 1H-indazole, 3-methyl- 0.58 Others methoxy-phenyl-oxime 3.95 2-nitrobenzaldoxime 1.78 2-propanone 8.14 propanal, 2-propenylhydrazone 1.33 methoxyphenyl-oxime 8.70 methylhydrazone -
[1] 朱开伟, 刘贞, 贺良萍, 林金钗.中国主要农作物秸秆可新型能源化生态经济总量分析[J].中国农业科学, 2016, 49(19):3769-3785. doi: 10.3864/j.issn.0578-1752.2016.19.009ZHU Kai-wei, LIU Zhen, HE Liang-ping, LIN Jin-chai. Eco-economic potential analysis of Chinese main crops' bio-energy utilization straw resouces[J]. Scientia Agricultra Sinica, 2016, 49(19):3769-3785. doi: 10.3864/j.issn.0578-1752.2016.19.009 [2] YIN C, ROSENDAHL L A, KÆR S K. Grate-firing of biomass for heat and power production[J]. Prog Energy Combust Sci, 2008, 34(6):725-754. doi: 10.1016/j.pecs.2008.05.002 [3] GLARBORG P, JENSEN A D, JOHNSSON J E. Fuel nitrogen conversion in solid fuel fired systems[J]. Prog Energy Combust Sci, 2003, 29(2):89-113. doi: 10.1016/S0360-1285(02)00031-X [4] 聂虎, 余春江, 柏继松, 李廉明, 秦建光, 方梦祥, 骆仲泱.生物质燃烧中硫氧化物和氮氧化物生成机理研究[J].热力发电, 2010, 39(9):21-26. http://www.doc88.com/p-287604204700.htmlNIE Hu, YU Chun-jiang, BAI Ji-song, LI Lian-ming, QIN Jian-guang, FANG Meng-xiang, LUO Zhong-yang. Study on formation mechanisms of sulphide and nitrogen oxides in combustion of biomass[J]. Therm Power Gen, 2010, 39(9):21-26. http://www.doc88.com/p-287604204700.html [5] CHEN H F, WANG Y, XU G W, YOSHIKAWA K. Fuel-N evolution during the pyrolysis of industrial biomass wastes with high nitrogen content[J]. Energies, 2012, 5(12):5418-5438. doi: 10.3390/en5125418 [6] TIAN F J, LI B Q, CHEN Y, LI C Z. Formation of NOx precursors during the pyrolysis of coal and biomass. Part Ⅴ. Pyrolysis of a sewage sludge[J]. Fuel, 2002, 81(17):2203-2208. doi: 10.1016/S0016-2361(02)00139-4 [7] HANSSON K M, SAMUELSSON J, TULLIN C, ÅMAND L E. Formation of HNCO, HCN, and NH3, from the pyrolysis of bark and nitrogen-containing model compounds[J]. Combust Flame, 2004, 137(3):265-277. doi: 10.1016/j.combustflame.2004.01.005 [8] TIAN Y, ZHANG J, ZUO W, CHEN L, CUI Y, TAN T. Nitrogen conversion in relation to NH3 and HCN during microwave pyrolysis of sewage sludge[J]. Environ Sci Technol, 2013, 47(7):3498-3505. doi: 10.1021/es304248j [9] CHEN W, YANG H P, CHEN Y Q, XIA M W, CHEN X, CHEN H P. Transformation of nitrogen and evolution of N-containing species during algae pyrolysis[J]. Environ Sci Technol, 2017, 51(11):6570-6579. doi: 10.1021/acs.est.7b00434 [10] GROTKJÆR T, DAM-JOHANSEN K, JENSEN A D, GLARBORG P. An experimental study of biomass ignition[J]. Fuel, 2003, 82(7):825-833. doi: 10.1016/S0016-2361(02)00369-1 [11] MOMENI M, YIN C, KÆR S K, HVID S L. Comprehensive study of ignition and combustion of single wooden particles[J]. Energy Fuels, 2013, 27(2):1061-1072. doi: 10.1021/ef302153f [12] 吴文广, 罗永浩, 陈祎, 陈亮, 王芸. 两段式固定床反应器中焦油脱除的实验研究[J]. 燃料化学学报, 2012, 40(2): 177-183. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=rlhx201202010&dbname=CJFD&dbcode=CJFQWU Wen-guang, LUO Yong-hao, CHEN Yi, SU Yi, CHEN Liang, WANG Yun. Experimental study on tar destruction in a two-stage fixed-bed reactor[J]. J Fuel Chem Technol, 2012, 40(2):177-183. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=rlhx201202010&dbname=CJFD&dbcode=CJFQ [13] 赵善辉, 罗永浩, 苏毅, 吴文广, 刘春元.部分氧化对焦油模型化合物苯酚转化的机理[J].化工学报, 2013, 64(10):3790-3796. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=hgsz201310046&dbname=CJFD&dbcode=CJFQZHAO Shan-hui, LUO Yong-hao, SU Yi, WU Wen-guang, LIU Chun-yuan. Reaction mechanism for partial oxidation of biomass tar[J]. J Chem Ind Eng (China), 2013, 64(10):3790-3796. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=hgsz201310046&dbname=CJFD&dbcode=CJFQ [14] DEBONO O, VILLOT A. Nitrogen products and reaction pathway of nitrogen compounds during the pyrolysis of various organic wastes[J]. J Anal Appl Pyrolysis, 2015, 114:222-234. doi: 10.1016/j.jaap.2015.06.002 [15] ZHAN H, YIN X L, HUANG Y Q, YUAN H Y, WU C Z. NOx precursors evolving during rapid pyrolysis of lignocellulosic industrial biomass wastes[J]. Fuel, 2017, 207:438-448. doi: 10.1016/j.fuel.2017.06.046 [16] BECIDAN M. Experimental studies on municipal solid waste and biomass pyrolysis[D]. Trondheim:Norwegian University of Science and Technology, 2007. http://www.diva-portal.org/smash/record.jsf?pid=diva2:122892 [17] RATCLIFF JR M A, MEDLEY E E, SIMMONDS P G. Pyrolysis of amino acids. Mechanistic considerations[J]. J Org Chem, 1974, 39(11):1481-1490. doi: 10.1021/jo00924a007 [18] LI J, WANG Z Y, YANG X, HU L, LIU Y W, WANG C X. Evaluate the pyrolysis pathway of glycine and glycylglycine by TG FT-IR[J]. J Anal Appl Pyrolysis, 2007, 80(1):247-253. doi: 10.1016/j.jaap.2007.03.001 [19] SAMUELSSON J I. Conversion of nitrogen in a fixed burning biofuel bed[D]. Go teborg:Chalmers University of Technology, 2006. https://core.ac.uk/display/70617058 [20] VOORHEES K J, ZHANG W, HENDRICKER A D, MURUGAVERL B. An investigation of the pyrolysis of oligopeptides by Curie-point pyrolysis-tandem mass spectrometry[J]. J Anal Appl Pyrolysis, 1994, 30(1):1-16. doi: 10.1016/0165-2370(94)00795-0 [21] CHOI S S, KO J E. Analysis of cyclic pyrolysis products formed from amino acid monomer[J]. J Chromatogr A, 2011, 1218(46):8443-8455. doi: 10.1016/j.chroma.2011.09.055 [22] SIMMONDS P G, MEDLEY E E, RATCLIFF M A, SHULMAN G P. Thermal decomposition of aliphatic monoaminomonocarboxylic acids[J]. Anal Chem, 1972, 44(12):2060-2066. doi: 10.1021/ac60320a040 [23] SHARMA R K, CHAN W G, SEEMAN J I, HAJALIGOL M R. Formation of low molecular weight heterocycles and polycyclic aromatic compounds (PACs) in the pyrolysis of α-amino acids[J]. J Anal Appl Pyrolysis, 2003, 66(1):97-121. http://www.sciencedirect.com/science/article/pii/S0165237002001080 -





 下载:
下载: