Effect of preparation parameters on the catalytic performance of hydrothermally synthesized Co3O4 in the decomposition of N2O
-
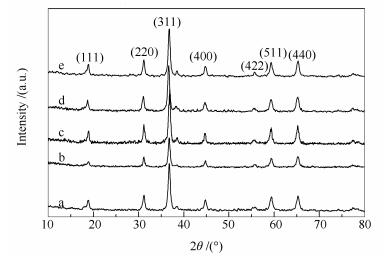
摘要: 以十六烷基三甲基溴化胺(CTAB)为模板剂,通过调变CTAB浓度水热合成了氧化钴前驱体,焙烧制得棒状形貌的Co3O4,在其表面浸渍K2CO3溶液制得K改性的Co3O4催化剂,用于N2O分解。用X射线衍射(XRD)、N2物理吸附(BET)、扫描电镜(SEM)、X射线光电子能谱(XPS)、H2程序升温还原(H2-TPR)和O2程序升温脱附(O2-TPD)等技术对催化剂进行了表征,考察了CTAB/钴及尿素/钴物质的量比等制备参数对Co3O4催化分解N2O活性的影响。结果表明,CTAB浓度为0.05 mol/L、CTAB/钴离子物质的量比为1、尿素/钴离子物质的量比为4时,所制备的Co3O4催化剂具有较高的N2O分解活性,而K改性可以进一步提升其催化性能。K改性的Co3O4在有氧有水气氛中400℃下进行N2O分解反应,50 h后N2O转化率仍保持在91%以上。Abstract: With hexadecyl trimethyl ammonium bromide (CTAB) as the template, cobaltosic oxide precursors were hydrothermally synthesized. Co3O4 catalysts were then prepared by calcining the cobaltosic oxide precursors, which was further modified by impregnation with K2CO3 solution and used in the decomposition of N2O. The catalysts were characterized by means of X-ray diffraction (XRD), nitrogen physisorption, scanning electron microscopy (SEM), X-ray photoelectron spectroscopy (XPS), hydrogen temperature-programmed reduction (H2-TPR), and oxygen temperature-programmed desorption (O2-TPD); the effect of CTAB concentration, CTAB/cobalt molar ratio and urea/cobalt molar ratio on the catalytic activity of Co3O4 was investigated. The results indicated that the Co3O4 catalyst prepared by using 0.05 mol/L CTAB solution, with a CTAB to cobalt molar ratio of 1 and a urea to cobalt molar ratio of 4, exhibits high activity in N2O decomposition. The catalytic performance of Co3O4 can be further enhanced by modifying with K. Over the 0.02 K/Co3O4 catalyst, the N2O conversion remains over 91% at 400 ℃ after conducting the N2O decomposition reaction for 50 h in the presence of oxygen and steam.
-
Key words:
- Co3O4 /
- hydrothermal synthesis /
- preparation parameters /
- catalytic decomposition /
- N2O
-
Table 1 BET surface area of various Co3O4 catalysts synthesized by changing the CTAB concentration
Catalyst BET surface area A/(m2·g-1) Co3O4(CTAB-0) 21.1 Co3O4(CTAB-0.01) 42.2 Co3O4(CTAB-0.03) 47.2 Co3O4(CTAB-0.05) 50.3 Co3O4(CTAB-0.06) 27.8 Table 2 Kinetic data for N2O decomposition over various Co3O4 catalysts synthesized by changing the CTAB concentration
Catalyst k/s-1 Ea/(kJ·mol-1) lnA 300 ℃ 325 ℃ 350 ℃ 375 ℃ 400 ℃ Co3O4(CTAB-0) - - 1.96 3.77 6.55 84.2 16.9 Co3O4(CTAB-0.01) 0.75 0.91 2.45 4.02 6.55 74.5 15.2 Co3O4(CTAB-0.03) 1.01 1.87 3.53 5.70 7.30 65.4 13.8 Co3O4(CTAB-0.05) 1.33 2.45 4.40 7.50 11.50 69.8 14.9 Co3O4(CTAB-0.06) 0.75 1.42 2.86 5.39 7.71 77.1 15.9 Table 3 Kinetic data of N2O decomposition over 0.02K/Co3O4 under different reaction atmospheres
Reaction feed k/s-1 Ea /(kJ·mol-1) lnA 250 ℃ 275 ℃ 300 ℃ 325 ℃ 1%N2O + Ar 2.76 7.50 13.09 20.10 68.2 16.8 1%N2O + 2%O2 + Ar 1.78 5.39 9.90 14.04 71.4 17.2 1%N2O + 2%O2 + 8.2%H2O + Ar 0.52 1.42 3.19 6.73 88.5 19.7 -
[1] LIN Y, MENG T, MA Z. Catalytic decomposition of N2O over RhOx supported on metal phosphates[J]. J Ind Eng Chem, 2015, 28:138-146. doi: 10.1016/j.jiec.2015.02.009 [2] PACHATOURIDOU E, PAPISTA E, DELIMITIS A, VASILIADES M A, EFSTATHIOU A M, AMIRIDIS M D, ALEXEEV O S, BLOOM D, MARNELLOS G E, KONSOLAKIS M, ILIOPOULOU E. N2O decomposition over ceria-promoted Ir/Al2O3 catalysts:The role of ceria[J]. Appl Catal B:Environ, 2016, 187:259-268. doi: 10.1016/j.apcatb.2016.01.049 [3] CARABINEIRO S A C, PAPISTA E, MARNELLOS G E, TAVARES P B, MALDONADO-HÓDAR F J, KONSOLAKIS M. Catalytic decomposition of N2O on inorganic oxides:Effect of doping with Au nanoparticles[J]. Mol Catal, 2017, 436:78-89. doi: 10.1016/j.mcat.2017.04.009 [4] XIE P F, MA Z, ZHOU H B, HUANG C Y, YUE Y H, SHEN W, XU H L, HUA W M, GAO Z. Catalytic decomposition of N2O over Cu-ZSM-11 catalysts[J]. Microporous Mesoporous Mater, 2014, 191:112-117. doi: 10.1016/j.micromeso.2014.02.044 [5] KUBONOVÁ L, PEIKERTOVÁ P, KUTLÁKOVÁ K M, JIRÁTOVÁ K, SŁOWIK G, OBALOVÁ L, COOL P. Catalytic activity of cobalt grafted on ordered mesoporous silicamaterials in N2O decomposition and CO oxidation[J]. Mol Catal, 2017, 437:57-72. doi: 10.1016/j.mcat.2017.04.037 [6] MELIÁN-CABRERA I, VAN ECK E R H, ESPINOSA S, SILES-QUESADA S, FALCO L, KENTGENS A P M, KAPTEIJN F, MOULIJN J A. Tail gas catalyzed N2O decomposition over Fe-beta zeolite. On the promoting role of framework connected AlO6 sites in the vicinity of Fe by controlled dealumination during exchange[J]. Appl Catal B:Environ, 2017, 203:218-226. doi: 10.1016/j.apcatb.2016.10.019 [7] SÁDOVSKÁ G, TABOR E, SAZAMA P, LHOTKA M, BERNAUER M, SOBALÍK Z. High temperature performance and stability of Fe-FER catalyst for N2O decomposition[J]. Catal Commun, 2017, 89:133-137. doi: 10.1016/j.catcom.2016.10.029 [8] MANIAK G, STELMACHOWSKI P, ZASADA F, PISKORZ W, KOTARBA A, SOJKA Z. Guidelines for optimization of catalytic activity of 3d transition metal oxide catalysts in N2O decomposition by potassium promotion[J]. Catal Today, 2011, 176:369-372. doi: 10.1016/j.cattod.2010.11.043 [9] LIU Z M, HE C X, CHEN B H, LIU H Y. CuO-CeO2 mixed oxide catalyst for the catalytic decomposition of N2O in the presence of oxygen[J]. Catal Today, 2017, 297:78-83. doi: 10.1016/j.cattod.2017.05.074 [10] LIU Z M, ZHOU Z Z, HE F, CHEN B H, ZHAO Y Y, XU Q. Catalytic decomposition of N2O over NiO-CeO2 mixed oxide catalyst[J]. Catal Today, 2017, 293/294:56-60. [11] CHROMCÁKOVÁ Z, OBALOVÁ L, KOVANDA F, LEGUT D, TITOV A, RITZ M, FRIDRICHOVÁ D, MICHALIK S, KUSTROWSKI P, JIRÁTOVÁ K. Effect of precursor synthesis on catalytic activity of Co3O4 in N2O decomposition[J[. Catal Today, 2015, 257:18-25. doi: 10.1016/j.cattod.2015.03.030 [12] OHNISHI C, ASANO K, IWAMOTO S, CHIKAMA K, INOUE M. Alkali-doped Co3O4 catalysts for direct decomposition of N2O in the presence of oxygen[J]. Catal Today, 2007, 120:145-150. doi: 10.1016/j.cattod.2006.07.042 [13] MANIAK G, STELMACHOWSKI P, KOTARBA A, SOJKA Z, RICO-PéREZ V, BUENO-LóPEZ A. Rationales for the selection of the best precursor for potassium doping of cobalt spinel based deN2O catalyst[J]. Appl Catal B:Environ, 2013, 136/137:302-307. doi: 10.1016/j.apcatb.2013.01.068 [14] GRZYBEK G, WÓJCIK S, CIURA K, GRYBO Ś J, INDYKA P, OSZAJCA M, STELMACHOWSKI P, WITKOWSKI S, INGER M, WILK M, KOTARBA A, SOJKA Z. Influence of preparation method on dispersion of cobalt spinel over alumina extrudates and the catalyst deN2O activity[J]. Appl Catal B:Environ, 2017, 210:34-44. doi: 10.1016/j.apcatb.2017.03.053 [15] YU H B, WANG X P, WU X X, CHEN Y. Promotion of Ag for Co3O4 catalyzing N2O decomposition under simulated real reaction conditions[J]. Chem Eng J, 2018, 334:800-806. doi: 10.1016/j.cej.2017.10.079 [16] WANG Y Z, HU X B, ZHENG K, WEI X H, ZHAO Y X. Effect of SnO2 on the structure and catalytic performance of Co3O4 for N2O decomposition[J]. Catal Commun, 2018, 111:70-74. doi: 10.1016/j.catcom.2018.04.004 [17] DOU Z, ZHANG H J, PAN Y F, XU X F. Catalytic decomposition of N2O over potassium-modified Cu-Co spinel oxides[J]. J Fuel Chem Technol, 2014, 42(2):238-245. doi: 10.1016/S1872-5813(14)60016-5 [18] LIU T Q, GUO R, SHEN M, YU W L. Determination of the diffusion coefficients of micelle and the first CMC and second CMC in SDS and CTAB solution[J]. Acta Phys Chim Sin, 1996, 12(4):337-340. [19] STELMACHOWSKI P, MANIAK G, KOTARBA A, SOJKA Z. Strong electronic promotion of Co3O4 towards N2O decomposition by surface alkali dopants[J]. Catal Commun, 2009, 10(7):1062-1065. doi: 10.1016/j.catcom.2008.12.057 [20] PAN Y F, FENG M, CUI X, XU X F. Catalytic activity of alkali metal doped Cu-Al mixed oxides for N2O decomposition in the presence of oxygen[J]. J Fuel Chem Technol, 2012, 40(5):601-607. doi: 10.1016/S1872-5813(12)60024-3 [21] FRANKEN T, PALKOVITS R. Investigation of potassium doped mixed spinels CuxCo3-xO4 as catalysts for an efficient N2O decomposition in real reaction conditions[J]. Appl Catal B:Environ, 2015, 176-177:298-305. doi: 10.1016/j.apcatb.2015.04.002 -





 下载:
下载: