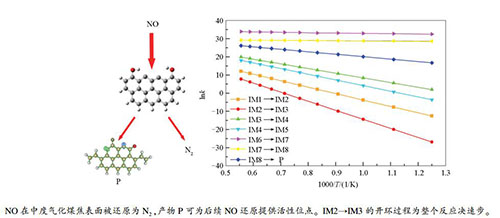
The mechanism of heterogeneous reduction reaction of NO by moderate gasification char
-
摘要: 采用量子化学密度泛函理论结合热力学和动力学研究了中度气化的锯齿形煤焦异相还原NO的反应机理。分析了中度气化煤焦异相还原NO的反应路径、异相还原过程中的能量变化以及热力学和动力学分析。结果表明,中度气化煤焦更易于NO的吸附,IM2→IM3的开环过程为整个反应的决速步,所需克服能垒最大(398.03 kJ/mol)。中度气化煤焦异相还原NO的反应在煤燃烧系统中为可自发的放热反应,且为单向反应。根据决速步理论,反应的进行需克服较大活化能(389.83 kJ/mol),同时根据阿伦尼乌斯公式,总体反应速率受温度影响较大,温度越高反应速率越快,越利于NO还原。Abstract: Zigzag carbonaceous model was applied to investigate the heterogeneous reduction mechanism of NO by moderate gasification char through the density functional theory in quantum chemistry method combined with thermodynamics and kinetics. The reaction path of heterogeneous reduction of NO by moderate gasification char were analyzed, and the energy change during heterogeneous reduction, thermodynamic and kinetic analysis were conducted. Research results show that the moderate gasification char is prone to adsorb NO. The process of CO desorption, which provides active sites for NO reduction, is a reaction rate determining step, and need to overcome the maximum barrier(398.03 kJ/mol). The reduction reaction is spontaneous and exothermic reaction in the coal combustion system and takes place in one direction. According to the theory of reaction rate determining step, the progress of the reaction need to overcome the larger activation energy(389.83 kJ/mol), and according to Arrhenius expression, the overall reaction rate is greatly affected by temperature. The higher the temperature is, the faster the reaction rate is, and the more favorable for NO reduction.
-
表 1 IM5轨道分析
Table 1 Orbital analysis of IM5
Species HOMO/eV LUMO/eV Gap/eV IM5 -4.843 -3.589 1.254 表 2 由NBO计算的一些重要原子的自然居群
Table 2 Natural population of some important atoms calculated by NBO
Atom Species Charge Valence C5 IM6 0.11055 1.87912 IM7 0.15988 1.82292 O* IM6 -0.14716 3.14068 IM7 -0.31445 3.30572 N* IM6 0.04322 2.44465 IM7 -0.05353 2.53722 N^ IM6 0.12199 3.15905 IM7 0.05894 2.42765 表 3 各步反应动力学参数
Table 3 Kinetic parameters for reaction steps
Reaction A Ea IM1→IM2 6.09×1013 294.9 IM2→IM3 2.5×1015 407.4 IM3→IM4 6.0×1014 212.76 IM4→IM5 2.5×1015 259.8 IM5+NO→IM6 - - IM6→IM7 4.1×1013 291.17 IM7→IM8 1.4×1015 408.8 IM8→P 6×1014 212.76 -
[1] 付兴民, 张玉秀, 郭战英, 刘海兵, 柳树成, 贾晋炜, 舒新前.炼焦煤尾煤热解特性及动力学研究[J].煤炭学报, 2013, 38(2):320-325. http://www.oalib.com/paper/4233736FU Xing-min, ZHANG Yu-xiu, GUO Zhan-ying, LIU Hai-bing, LIU Shu-cheng, JIA Jin-wei, SHU Xin-qian. Characteristics andkinetics of the pyrolysis of coking coal tailings[J]. J China Coal Soc, 2013, 38(2):320-325. http://www.oalib.com/paper/4233736 [2] ZHENG M, LI X, LIU J, GUO L. Initial chemical reaction simulation of coal pyrolysis via reaxff molecular dynamics[J]. Energy Fuels, 2013, 27(6):2942-2951. doi: 10.1021/ef400143z [3] 苏亚欣, 苏阿龙, 成豪.金属铁直接催化还原NO的实验研究[J].煤炭学报, 2013, 38(S1):206-210. http://www.actasc.cn/hjkxxb/ch/reader/view_abstract.aspx?file_no=20170514003&flag=1SU Ya-xin, SU A-long, CHENG Hao. Experimental study on direct catalytic reduction of NO by metallic iron[J]. J China Coal Soc, 2013, 38(S1):206-210. http://www.actasc.cn/hjkxxb/ch/reader/view_abstract.aspx?file_no=20170514003&flag=1 [4] SUZUKI T, KYOTANI T, TOMITA A. Study on the carbon-nitric oxide reactionin the presence of oxygen[J]. Ind Eng Chem Res, 1994, 33:2840-2845. doi: 10.1021/ie00035a038 [5] YAMASHITA H, TOMITA A, YAMADA H, KYOTANI T, RADOVIC L R. Influence of char surfacechemistry on the reduction of nitric oxide with chars[J]. Energy Fuels, 1993, 7:85-89. doi: 10.1021/ef00037a014 [6] GUPTA H, FAN L-S. Reduction of nitric oxide from combustion flue gas bybituminous coal char in the presence of oxygen[J]. Ind Eng Chem Res, 2003, 42:2536-2543. doi: 10.1021/ie020693n [7] XIN J, SUN B M, ZHU H Y, YIN S J, ZHANG Z X, ZHONG Y F. Variation analysis of Mayer bond order during the heterogeneous reduction reaction between NO and char edge models[J]. J China Coal Soc, 2014, 39(4):771-775. [8] ZHOU Z, ZHANG X, ZHOU J, LIU J, CEN K. A molecular modeling study of N2 desorption from NO heterogeneous reduction on char[J]. Energy Source, 2014, 36(2):158-166. doi: 10.1080/15567036.2010.506477 [9] ZHU H Y, SUN B M, XINJ, YIN S J, XIAO H P. Quantum chemistry research on NO heterogeneous reduction by char with the participation of CO under oxy-fuel combustion atmosphere[J]. J China Coal Soc, 2015, 40(7):1641-1647. https://www.researchgate.net/publication/282931204_Quantum_chemistry_research_on_NO_heterogeneous_reduction_by_char_with_the_participation_of_CO_under_oxy-fuel_combustion_atmosphere [10] ZHANG H, LIU J, WANG X, JIANG X. Density functional theory study on two different oxygen enhancement mechanisms during NO-char interaction[J]. Combust Flame, 2016, 169:11-18. doi: 10.1016/j.combustflame.2016.03.023 [11] 高正阳, 杨维结, 阎维平.煤焦催化HCN还原NO的反应机理[J].燃料化学学报, 2017, 45(9):1043-1048. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract19083.shtmlGAO Zheng-yang, YANG Wei-jie, YAN Wei-ping. Reaction mechanism of NO reduction with HCN catalyzed by char[J]. J Fuel Chem Technol, 2017, 45(9):1043-1048. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract19083.shtml [12] KARINA S, BRIAN S H. Density functional study of the chemisorption of O2 on the zig-zag surface of graphite[J]. Combust Flame, 2005, 143(4):629-643. doi: 10.1016/j.combustflame.2005.08.026 [13] SENDT K, HAYNES B S. Density functional study of the reaction of carbon surface oxides:the behavior of ketones[J]. J Phys Chem A, 2005, 109(15):3438-3447. doi: 10.1021/jp045111p [14] SENDT K, HAYNES B S. Density functional study of the chemisorption of O2 across two rings of the armchair surface of graphite[J]. J Phys Chem C, 2007, 111(14):5465-5473. doi: 10.1021/jp067363r [15] ZHANG H, JIANG X, LIU J, SHEN J. Application of density functional theory to the nitric oxide heterogeneous reduction mechanism in the presence of hydroxyl and carbonyl groups[J]. Energy Convers Manage, 2014, 83(83):167-176. [16] YANG F H, YANG R T. Ab initio molecular orbital study of adsorption of atomic hydrogen on graphite:Insight into hydrogen storage in carbon nanotubes[J]. Carbon, 2002, 40(3):437-444. doi: 10.1016/S0008-6223(01)00199-3 [17] CHEN N, YANG R T. Ab initio molecular orbital calculation on graphite:Selection of molecular system and model chemistry[J]. Carbon, 1998, 36(7):1061-1070. [18] MIN J X, WANG N B, WANG M F, HUO P J, LIU D. Investigation on the catalytic effects of AAEM during steam gasification and the resultant char reactivity in oxygen using Shengli lignite at different forms[J]. Int J Coal Sci Technol, 2015, 2(3):223-231. doi: 10.1007/s40789-015-0083-0 [19] LI H B, YU Y, HAN M F, LEI Z. Simulation of coal char gasification using O2/CO2[J]. Int J Coal Sci Technol, 2014, 1(1):81-87. doi: 10.1007/s40789-014-0010-9 [20] 钟俊, 高正阳, 丁艺, 余岳溪, 杨维结. Zigzag煤焦表面异相还原N2O反应[J].煤炭学报, 2017, 42(11):3028-3034.ZHONG Jun, GAO Zheng-yang, DING Yi, YU Yue-xi, YANG Wei-jie. Heterogeneous reduction reaction of N2O by char based on Zigzag carbonaceous model[J]. J China Coal Soc, 2017, 42(11):3028-3034. [21] 张秀霞. 焦炭燃烧过程中氮转化机理与低NOx燃烧技术的开发[D]. 浙江: 浙江大学, 2012.ZHANG Xiu-xia. Nitrogen conversion mechanism during char combustion and develepment of low NOx technology[D]. Zhejiang: Zhejiang University, 2012. [22] SENDT K, HAYNES B S. Density functional study of the reaction of O2 with a single site on the zigzag edge of graphene[J]. Proc Combust Inst, 2011, 33(2):1851-1858. doi: 10.1016/j.proci.2010.06.021 [23] PHAM B Q, TRUONG T N. Electronic spin transitions in finite-size graphene[J]. Chem Phys Lett, 2012, 535(7):75-79. [24] ALEJANDRO M, THANH-THAI T T, FANOR M, THANH N. T. CO desorption from oxygen species on carbonaceous surface:1. Effects of the local structure of the active site and the surface coverage[J]. J Phys Chem A, 2001, 105(27):6757-6764. doi: 10.1021/jp010572l [25] FRISCH M J, TRUCKS G W, SCHLEGEL H B, SCUSERIA G E, ROBB M A, et al. Gaussian09, revision E. 01[J]. Gaussian Inc., Wallingford, CT, 2009. http://www.docin.com/p-1716228155.html [26] ZHANG H, LIU J, SHEN J, JIANG X. Thermodynamic and kinetic evaluation of the reaction between NO (nitric oxide) and char(N) (char bound nitrogen) in coal combustion[J]. Energy, 2015, 82:312-21. doi: 10.1016/j.energy.2015.01.040 [27] ALI M A, RAJAKUMAR B. Thermodynamic and kinetic studies of hydroxyl radical reaction with bromine oxide using density functional theory[J]. Comput Theor Chem, 2011, 964:283-290. doi: 10.1016/j.comptc.2011.01.013 [28] PEVIDA C, ARENILLAS A, RUBIERA F, PIS J J. Synthetic coal chars for the elucidation of NO heterogeneous reduction mechanisms[J]. Fuel, 2007, 86:41-49. doi: 10.1016/j.fuel.2006.07.002 [29] PEVIDA C, ARENILLAS A, RUBIERA F, PIS J J. Heterogeneous reduction of nitric oxide on synthetic coal chars[J]. Fuel, 2005, 84:2275-2279. doi: 10.1016/j.fuel.2005.06.003 [30] GAO Z Y, LV S K, YANG W J, YANG P F, JI S, MENG X X. Quantum chemistry investigation on the reaction mechanism of the elemental mercury, chlorine, bromine and ozone system[J]. J Mol Model, 2015, 21(6):1-9. -





 下载:
下载: