Performance of various catalysts in hydropyrolysis of organic matters and reaction mechanisms
-
摘要: 对不同类型催化剂如ZnCl2、NiCl2、Fe2O3、Y型沸石(NaY) 及MoS2上有机质的催化加氢热解行为及反应机理进行了研究。结果表明, 有机质加氢液态产物收率和组成受控于催化剂类型, 但不同催化剂上加氢热解所得到的产物生标参数差异不大; 同时, 对于不同成熟度和类型的有机质样品, 各催化剂所体现的催化效果也不尽相同。基于固体残渣元素组成、红外光谱和X射线衍射分析结果发现, 不同类型催化剂上的加氢作用机制也存在明显差异。与NiCl2相比, ZnCl2体系中除存在催化裂解和催化加氢作用外, 还存在质量传递效应; Fe2O3催化剂主要是通过其表面的活性O吸收H2中的H形成H自由基而加速有机质的加氢反应; MoS2体系存在过渡金属Mo的催化加氢和中间产物H2S的自由基引发作用两种催化机制。Abstract: The hydropyrolysis of organic matters was comparatively conducted over various catalysts including ZnCl2, NiCl2, Fe2O3, NaY and MoS2 and the reaction mechanisms over different catalysts were then investigated. The results indicate that the yield and composition of liquid products from hydropyrolysis are related to the type of catalyst, though various catalysts do not have significant difference in the product biomarker parameters. Meanwhile, for the organic matters with different maturities and types, various catalysts are also quite different in their actual performance. The element analysis, infrared spectra and X-ray diffractograms of the solid residues illustrate that various catalysts are obviously different in the catalytic reaction mechanism. In comparison with NiCl2, mass transfer is an important factor in the ZnCl2 system, besides the catalytic cracking and catalytic hydrogenation. With Fe2O3 as the catalyst, the formation of H free radical by the adsorption of H2 at the surface active O sites may promote the hydrogenation of organic matters. MoS2 as the catalyst may involve two mechanisms, viz., the hydrogenation over transition metal Mo and the initiation of free radicals from H2S intermediates.
-
Key words:
- organic matters /
- hydropyrolysis /
- biomarkers /
- catalytic reaction mechanism
-
表 1 催化加氢热解样品的地球化学特征
Table 1 Geochemical characteristic of the organic matters for catalytic hydropyrolysis
Sample Depth L/m Layer TOC w/% R0 w/% Elemental compostion of kerogen w/% C H O N Du-13 1 408.5 K 3.14 0.84 60.44 1.231 6.61 1.65 Zhaoshen-5 4 331 C-P - 2.06 72.99 0.296 2.17 1.83 Fuxin coal surface J 71.24 0.55 73.52 5.68 - 1.23 表 2 不同催化剂条件下有机质加氢热解的液体产物产率及组成特征
Table 2 Yields and compositions of liquid products from catalytic hydropyrolysis of organic matters over different catalysts
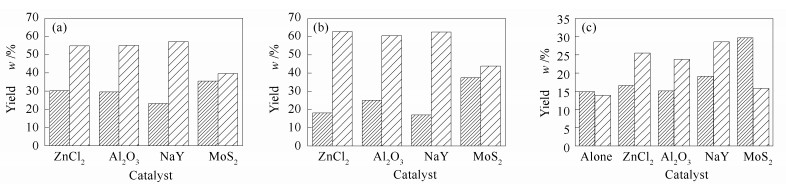
Sample Catalyst Overall yield of liquid hydrocarbons w/(mg·g-1) Group composition w/% saturates aromatics NSO compounds and asphaltenes Kerogen of Du-13 ZnCl2 292.0 30.2 54.8 15.0 Al2O3 258.0 29.6 55.1 15.3 NaY 298.4 23.2 57.1 19.7 MoS2 390.9 35.5 39.6 24.9 Kerogen of Zhaoshen-5 ZnCl2 40.2 18.1 62.8 19.1 Fe2O3 16.0 25.0 60.5 14.5 Al2O3 17.6 17.0 62.5 20.5 MoS2 18.0 37.5 43.8 18.7 Fuxin coal alone 162.8 14.9 13.9 71.2 ZnCl2 606.0 16.6 25.5 57.9 NiCl2 541.5 15.1 23.8 61.1 Fe2O3 503.6 19.1 28.6 60.3 MoS2 237.8 29.7 15.8 54.5 表 3 阜新煤不同催化加氢热解产物的生标参数
Table 3 Biomarker parameters of pyrolysis products from Fuxin coal over different catalysts
Biomarker parameter Extracts* Catalyst free Catalytic conditions ZnCl2 NiCl2 Fe2O3 MoS2 Pr/Ph 7.85 5.47 6.30 4.13 4.32 4.70 Pr/ nC17 4.01 0.68 1.23 0.59 0.51 0.93 Ph/ nC18 0.57 0.12 0.21 0.14 0.11 0.19 CPI 1.87 1.20 1.43 1.22 1.15 1.21 OEP-1 1.34 1.05 1.13 1.06 1.04 1.06 C22/C21 tricyclic terpane 0.43 0.37 0.47 0.34 0.32 0.32 Ts/(Ts+Tm) 0.36 0.33 - 0.23 0.22 0.29 C29 βα /C29 αβ 0.52 0.38 0.45 0.35 0.37 0.42 C30 βα /C30 αβ 0.48 0.41 0.50 0.45 0.46 0.51 C31αβ 22S/(22S+22R) 0.57 0.52 0.49 0.51 0.48 0.46 C32αβ 22S/(22S+22R) 0.56 0.53 0.48 0.50 0.43 0.47 C29 20S/(20S+20R) 0.23 0.26 0.22 0.25 0.24 0.19 C29 ββ/(ββ+αα) 0.23 0.24 0.20 0.24 0.15 0.15 MPI-1 0.33 0.71 0.56 0.65 0.62 0.58 MNR 1.08 2.37 2.16 2.54 2.08 2.00 ENR 0.23 0.24 0.39 0.37 0.44 0.40 *: extracts represent the liquid products obtained by extraction of organic matters 表 4 阜新煤样加氢热解后固体残余物的元素分析
Table 4 Elemental compositions of solid residues
Reaction condition Content w/% C/H* C H O S N Catalyst free 77.04 4.32 6.94 1.02 1.39 1/1.75 ZnCl2 63.57 3.12 5.84 1.43 0.96 1/1.13 NiCl2 62.88 3.17 4.30 1.03 1.03 1/1.20 Fe2O3 45.76 2.34 3.66 1.40 0.82 1/1.11 MoS2 72.39 5.09 6.89 2.81 1.48 1/1.07 *: C/H represents the ratio of carbon conversion to hydrogen in the hydropyrolysis -
[1] 李保庆.我国煤加氢热解研究Ⅱ.先锋褐煤加氢及催化加氢热解的热重研究[J].燃料化学学报, 1995, 23(2): 186-191. http://www.cnki.com.cn/Article/CJFDTOTAL-RLHX502.012.htmLI Bao-qing. Hydropyrolysis of Chinese coals Ⅱ. Thermogravimetric study on catalytic and non-catalytic hydropyrolysis of Xianfeng lignite[J]. J Fuel Chem Technol, 1995, 23(2): 186-191. http://www.cnki.com.cn/Article/CJFDTOTAL-RLHX502.012.htm [2] 李保庆.我国煤加氢热解研究Ⅲ.神府煤加氢、催化加氢及H2-CH4气氛下热解的研究[J].燃料化学学报, 1995, 23(2): 192-196. http://www.cnki.com.cn/Article/CJFDTOTAL-RLHX502.012.htmLI Bao-qing. Hydropyrolysis of Chinese coals Ⅲ. Catalytic and non-catalytic hydropyrolysis under H2-CH4 of Shenfu bituminous coal[J]. J Fuel Chem Technol, 1995, 23(2): 192-196. http://www.cnki.com.cn/Article/CJFDTOTAL-RLHX502.012.htm [3] PETERS K E, MOLDOWAN J M. The bionmarker guide: Interpreting molecular fossoils in petroleum and ancient sediments[M]. New Jersey: Prentice Hall, 1993: 170-176. [4] 傅家谟, 盛国英, 许家友, 贾蓉芬, 范善发, 彭平安, EGLINTON G, GOWAR A P.应用生物标志化合物参数判识古沉积环境[J].地球化学, 1991, 20(1): 1-12. http://www.cnki.com.cn/Article/CJFDTOTAL-DQHX199101000.htmFU Jia-mo, SHENG Guo-ying, XU Jia-you, JIA Rong-fen, FAN Shan-fa, PENG Ping-an, EGLINTON G, GOWAR A P. Application of biomarker compounds in assessment of paleoenvironxnents of Chinese terrestrial sediments[J]. Geochimica, 1991, 20(1): 1-12. http://www.cnki.com.cn/Article/CJFDTOTAL-DQHX199101000.htm [5] LOVE G D, SNAPE C E, CAM A D. Release of covalently-bound alkane biomarkers in high yields from kerogen via catalytic hydropyrolysis[J]. Org Geochem, 1995, 23(10): 981-986. doi: 10.1016/0146-6380(95)00075-5 [6] IKENAGA N, KAN-NAN S, SAKODA T, SUZUKI T. Coal hydroliquefaction using highly dispersed catalyst precursors[J]. Catal Today, 1997, 39(1/2): 99-109. https://www.researchgate.net/publication/244320836_Coal_hydroliquefaction_using_highly_dispersed_catalyst_precursors [7] INUKAI Y. Hydroliquefaction of Illinois NO.6 coal with petroleum atmospheric residue using oil-soluble molybdenum catalyst[J]. Fuel Process Technol, 1995, 43(2): 157-167. doi: 10.1016/0378-3820(95)00017-2 [8] 岳长涛, 李术元, 徐明, 钟宁宁.柴油与硫酸镁反应体系模拟实验研究[J].石油实验地质, 2010, 32(6): 610-614. http://www.cnki.com.cn/Article/CJFDTOTAL-SYSD201006020.htmYUE Chang-tao, LI Shu-yuan, XU Ming, ZHONG Ning-ning. Simulation experiments on the TSR system of diesel and mangnesium sulfate[J]. Pet Geol Exp, 2010, 32(6): 610-614. http://www.cnki.com.cn/Article/CJFDTOTAL-SYSD201006020.htm [9] ROCHA J D, BROWN S D, LOVE G D, SNAPE C E. Hydropyrolysis: A versatile technique for solid fuel liquefaction, sulfur speciation and biomarker release[J]. J Anal Appl Pyrolysis, 1997, 40-41: 91-103. doi: 10.1016/S0165-2370(97)00041-7 [10] RUSSELL C A, SNAPE C E, MEREDITH W. The potential of bound biomarker prfiles released from catalytic hydropymlysis to reconstruction basin charging history for oils[C]. Abstract for 21th International Meeting on Organic Geochemistry K rakow. 2003: 160-161. [11] LOCKHART R S, MEREDIT H W, LOVE G D. Release of bound aliphatic biomarkers via hydropyrolysis from Type Ⅱ kerogen at high maturity[J]. Org Geochem, 2008, 39(8): 1119-1124. doi: 10.1016/j.orggeochem.2008.03.016 [12] HE K, ZHANG S C, MI J K. Mechanism of catalytic hydropyrolysis of sedimentary organic matter with MoS2[J]. Pet Sci, 2011, 8(2): 134-142. doi: 10.1007/s12182-011-0126-0 [13] ZELENSKI C M, DORHOUT P K. Template synthesis of near-monodisperse microscale nanofibers and nanotubes of MoS2[J]. J Am Chem Soc, 1998, 120(4): 734-742. doi: 10.1021/ja972170q [14] FARAG H, EI-HEDAWY A, SAKAISHI K, KISHIDA M, MOCHIDA I. Catalytic activity of synthesized nanosized molybdenum disulfide for the hydrodesulfurization of dibenzthiophene: Effect of H2S partial pressure[J]. Appl Catal B: Environ, 2009, 91(1/2): 189-197. [15] SONG J H, CHEN P L, KIM S H. Catalytic cracking of n-hexane over MoO2[J]. J Mol Catal A, 2002, 184(1/2): 197-202. http://www.deepdyve.com/lp/elsevier/catalytic-cracking-of-n-hexane-over-moo-2-TZvu2LB0vd [16] PINTO F, GULYURTLU I, LOBO L S, CABRITA I. The effect of catalyst blending on coal hydropyrolysis[J]. Fuel, 1999, 78(7): 761-768. doi: 10.1016/S0016-2361(98)00212-9 [17] SONG C S, NOMURA M, MIYAKE M. Coal hydroliquefaction using MoCl3-and NiCl2-containing salts as catalysts: Difference in catalysis between solid and molten catalysis[J]. Fuel, 1986, 65(7): 922-926. doi: 10.1016/0016-2361(86)90199-7 [18] WANG L, CHEN P. Mechanism study of iron-based catalysts in co-liquefaction of coal with waste plastics[J]. Fuel, 2002, 81(6): 811-815. doi: 10.1016/S0016-2361(01)00201-0 [19] HE K, DONG Y M, LI Z. Catalytic ozonation of phenol in water with natural brucite and magnesia[J]. J Hazard Mater, 2008, 159(2/3): 587-592. https://www.researchgate.net/publication/5453098_Catalytic_ozonation_of_phenol_in_water_with_natural_brucite_and_magnesia [20] HE K, DONG Y M, LIN Y. A facile hydrothermal method to synthesize nanosized Co3O4/CeO2 and study of its catalytic characteristic in catalytic ozonation of phenol[J]. Catal Lett, 2009, 133(1): 209-213. [21] PRIYANTO U, SAKANISHI K, OKUMA O, MOCHIDA I. Liquefaction of Tanito Harum coal with bottom recycle using FeNi and FeMoNi catalysts supported on carbon nanoparticles[J]. Fuel Process Technol, 2002, 79(1): 51-62. doi: 10.1016/S0378-3820(02)00101-7 [22] SONG C, SAINI A K, YONEYAMA Y. A new process for catalytic liquefaction of coal using dispersed MoS2 catalytic generated in situ with added H2O[J]. Fuel, 2000, 79(3/4): 249-261. https://www.researchgate.net/publication/239142126_New_process_for_catalytic_liquefaction_of_coal_using_dispersed_MoS2_catalyst_generated_in_situ_with_added_H2O [23] BOONE W P, EKERDT J G. Hydrodesulfurization studies with a single-layer molybdenum disulfite catalyst[J]. J Catal, 2000, 193(1): 96-102. doi: 10.1006/jcat.2000.2884 [24] ZHANG T W, AMRANI A, ELLIS G S, MA Q S, TANG Y C. Experimental investigation on thermochemical sulfate reduction by H2S initiation[J]. Geochim Cosmochim Acta, 2008, 72(14): 3518-3530. doi: 10.1016/j.gca.2008.04.036 -






 下载:
下载: