Comparison of leaching behaviors of aluminum in ash from combustion and catalytic gasification
-
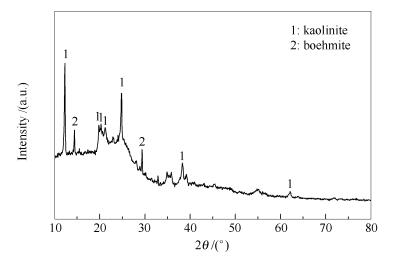
摘要: 实验对比了燃烧灰与负载Na2CO3催化气化灰中Al的溶出行为,考察了Na2CO3负载量(0-15%,质量分数)和温度(600-1 000℃)对催化气化灰中主要矿物组成与Al溶出行为的影响。同时,采用XRD分析了燃烧灰、催化气化灰以及酸浸残渣的主要组成。结果表明,燃烧灰的主要组成为莫来石,而催化气化灰的主要组成为硅铝酸钠((Na2O)0.33NaAlSiO4)。6 mol/L硫酸、60℃和30 min浸取条件下,燃烧灰的浸出率只有40%,而Na2CO3负载量为10%的催化气化灰的浸出率达到88%。催化气化灰更易于回收灰中的Al。Abstract: The leaching behaviors of aluminum in ash from combustion and catalytic gasification, and effects of Na2CO3 addition (0-15%), temperature (600-1 000℃) on Al leaching behaviors and mineral composition were investigated. The compositions of ash from combustion and gasification, and acid leaching residue were investigated by XRD. The results shows that the combustion ash is predominantly composed of mullite, while that from catalytic gasification is sodium aluminum silicate ((Na2O)0.33NaAlSiO4). The Al extraction yield of combustion ash only reaches 40% at leaching conditions of 6 mol/L H2SO4, 60℃ and 30 min, while that of catalytic gasification ash with 10% Na2CO3 addition can reach 88%. The catalytic gasification with Na2CO3 addition can achieve higher Al extraction yield.
-
Key words:
- catalytic gasification /
- coal ash /
- alumina extraction
-
表 1 孙家豪煤的工业分析和元素分析
Table 1 Proximate and ultimate analyses of SJH coal
Sample Proximate analysis wad/% Ultimate analysis wdaf/% V FC A M C H N S O* SJH coal 29.6 51.2 16.9 2.3 78.8 4.9 1.5 0.8 14 *: by difference 表 2 孙家豪煤灰的矿物质组成
Table 2 Ash compositions of SJH coal
Composition w/% Al2O3 SiO2 Fe2O3 TiO2 CaO MgO Na2O SO3 K2O P2O5 46.34 36.29 6.38 3.57 2.61 2.18 0.76 0.45 0.42 0.03 -
[1] 杜淄川, 李会泉, 李少鹏. 高铝粉煤灰碱溶脱硅过程反应机理[J]. 过程工程学报, 2011, 11(3):442-447. http://www.cnki.com.cn/Article/CJFDTOTAL-HGYJ201103015.htmDU Zi-chuan, LI Hui-quan, LI Shao-peng. Reaction mechanism of desilification process of high aluminum fly ash by alkali solution[J]. Chin J Process Eng, 2011, 11(3):442-447. http://www.cnki.com.cn/Article/CJFDTOTAL-HGYJ201103015.htm [2] NEUPANE G, DONAHOE R J. Leachability of elements in alkaline and acidic coal fly ash samples during batch and column leaching tests[J]. Fuel, 2013, 104:758-770. doi: 10.1016/j.fuel.2012.06.013 [3] PARK H C, PARK Y J, STEVENS R. Synthesis of alumina from high purity alum derived from coal fly ash[J]. Mater Sci Eng, 2004, 367(1/2):166-170. http://www.sciencedirect.com/science/article/pii/S0921509303010736 [4] YAO Z T, JI X S, SARKER P K, TANG J H, GE L Q, XIA M S, XI Y Q. A comprehensive review on the applications of coal fly ash[J]. Earth-Sci Rev, 2015, 141:105-121. doi: 10.1016/j.earscirev.2014.11.016 [5] 王蕾, 马鸿文. 利用粉煤灰制备高比表面积二氧化硅的实验研究[J]. 硅酸盐通报, 2006, (2):1-7. http://www.cnki.com.cn/Article/CJFDTOTAL-GSYT200602001.htmWANG Lei, MA Hong-wen. Experimental research on preparation of high specific surface area SiO2 from fly ash[J]. Bull Chin Ceram Soc, 2006, (2):1-7. http://www.cnki.com.cn/Article/CJFDTOTAL-GSYT200602001.htm [6] SHEMI A, MPANA R N, NDLOVU S, VANDYK L D, SIBANBA V, SEEPE L. Alternative techniques for extracting alumina from coal fly ash[J]. Miner Eng, 2012, 34:30-37. doi: 10.1016/j.mineng.2012.04.007 [7] WU C Y, YU H F, ZHANG H F. Extraction of aluminum by pressure acid-leaching method from coal fly ash[J]. Trans Nonferrous Met Soc, China, 2012, 22(9):2282-2288. doi: 10.1016/S1003-6326(11)61461-1 [8] 薄春丽, 郑诗礼. 高铝粉煤灰铝硅化合物在稀碱溶液中的浸出行为[J]. 过程工程学报, 2012, 12(4):613-617. http://www.cnki.com.cn/Article/CJFDTOTAL-HGYJ201204017.htmBO Chun-li, ZHENG Shi-li. Leaching behaviors of aluminum and silicon compounds in aluminum-rich fly ash in dilute alkaline solution[J]. Chin J Process Eng, 2012, 12(4):613-617. http://www.cnki.com.cn/Article/CJFDTOTAL-HGYJ201204017.htm [9] 佟志芳, 邹燕飞, 李英杰. 从粉煤灰提取铝铁新工艺研究[J]. 轻金属, 2009, 1(1):13-16. http://www.cnki.com.cn/Article/CJFDTOTAL-QJSS200901005.htmDONG Zhi-fang, CHUN Yan-fei, LI Ying-jie. Experimental study on extraction of aluminium ferric from coal fly ash[J]. Light Metal, 2009, 1(1):13-16. http://www.cnki.com.cn/Article/CJFDTOTAL-QJSS200901005.htm [10] YAO Z T, XIA M S, SARKER P K, CHEN T. A review of the alumina recovery from coal fly ash with a focus in China[J]. Fuel, 2014, 120:74-85. doi: 10.1016/j.fuel.2013.12.003 [11] WANG R C, ZHAI Y C, NING Z Q. Thermodynamics and kinetics of alumina extraction from fly ash using an ammonium hydrogen sulfate roasting method[J]. Int J Miner Metal Mater, 2014, 21(2):144-149. doi: 10.1007/s12613-014-0877-x [12] GUO Y, YAN K, CUI L, CHENG F, LOU H H. Effect of Na2CO3 additive on the activation of coal gangue for alumina extraction[J]. Inter J Miner Process, 2014, 131:51-57. doi: 10.1016/j.minpro.2014.07.001 [13] GUO Y, LI Y, CHENG F, WANG M, WANG X. Role of additives in improved thermal activation of coal fly ash for alumina extraction[J]. Fuel Process Technol, 2013, 110:114-121. doi: 10.1016/j.fuproc.2012.12.003 [14] 毛燕东, 金亚丹, 李克忠, 毕继诚, 李金来, 辛峰. 煤催化气化条件下不同煤种煤烧结行为研究[J]. 燃料化学学报, 2015, 43(4):402-409. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18602.shtmlMAO Yan-dong, JIN Ya-dan, LI Ke-zhong, BI Ji-cheng, LI Jin-lai, XIN Feng. Sintering behaviors of different coal ashes in catalytic gasification process[J]. J Fuel Chem Technol, 2015, 43(4):402-409. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18602.shtml [15] 卫俊涛, 丁路, 周志杰, 于广锁. 负载碳酸钾煤焦-CO2催化气化反应特性的原位研究[J]. 燃料化学学报, 2015, 43(11):1311-1319. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18724.shtmlWEI Jun-tao, DING Lu, ZHOU Zhi-jie, YU Guang-suo. In-situ analysis of catalytic gasification reaction characteristics of coal char-CO2 with K2CO3 additive[J]. J Fuel Chem Technol, 2015, 43(11):1311-1319. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18724.shtml [16] DING L, ZHANG Y, WANG Z, HUANG J, FANG Y. Interaction and its induced inhibiting or synergistic effects during co-gasification of coal char and biomass char[J]. Bioresour Technol, 2014, 173:11-20. doi: 10.1016/j.biortech.2014.09.007 [17] WANG Y, WANG Z, HUANG J, FANG Y. Catalytic gasification activity of Na2CO3 and comparison with K2CO3 for a high-aluminum coal char[J]. Energy Fuels, 2015, 29(11):6988-6998. doi: 10.1021/acs.energyfuels.5b01537 -





 下载:
下载: