Upgrading of coal tar with steam catalytic cracking over Al/Ce and Al/Zr co-doped Fe2O3 catalysts
-
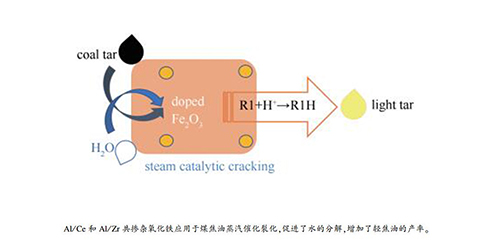
摘要: 蒸汽催化裂化(SCC)为煤焦油的提质提供了一种重要的方法。本研究以Al/Ce和Al/Zr共掺杂Fe2O3为催化剂研究了其在反应温度550 ℃、反应时间1 h下蒸汽催化裂化提质煤焦油的性能。催化剂表征显示掺杂的Fe2O3催化剂具有较小的晶粒粒径、较大的比表面积和孔体积。XPS表征表明,晶格氧是主要的活性氧物种,掺杂可以增加O-的浓度。催化蒸汽裂化结果表明,Al/Ce和Al/Zr共掺杂可以提高Fe2O3催化活性。轻焦油(沸点低于360 ℃)在FeAlZr1、FeAlZr2、FeAlCe1和FeAlCe2上的产率分别为63.2%、58.1%、60.2%和55.1%,高于Fe2O3上的产率49.7%。来自水蒸气解离和催化剂中的活性氧共同参与了煤焦油的改质。催化剂的比表面积和O-含量是决定蒸汽催化裂化性能的主要因素。Abstract: The upgrading of coal tar by means of steam catalytic cracking (SCC) is a promising method. In this study, Al/Ce and Al/Zr co-doped Fe2O3 catalysts were prepared and used for SCC of coal tar for improving the light tar yield. The SCC was conducted at 550 ℃ for 1 h. It was found that the crystal size decreased over doped Fe2O3 catalysts, and the pore volume and specific surface area increased. XPS analysis showed that lattice oxygen was the majority oxygen species and doping can increased the O- concentration. It was shown that Al/Ce and Al/Zr co-doped Fe2O3 could improve the catalytic activity of Fe2O3. The light tar yield over FeAlZr1, FeAlZr2, FeAlCe1 and FeAlCe2 were 63.2%, 58.1%, 60.2% and 55.1%, respectively, higher than that on Fe2O3 being 49.7%. The oxygen species from steam dissociation and Fe2O3 could take part in the upgrading of coal tar. It was revealed that the specific surface area and the O- on the Fe2O3 catalysts were the primary factors in determining the SCC performance.
-
Key words:
- coal tar /
- steam catalytic upgrading /
- co-doped Fe2O3
-
Table 1 Basic properties of coal tar[16]
Mna Content w/% Elemental analysis w/% H/C
atomic ratiowater pitch C H N S Ob 275 1.02 43.0 83.06 9.63 0.69 0.16 6.46 1.39 a: number average molecular weight; b: by difference Table 2 Structural parameters of co-doped and undoped Fe2O3 catalysts
Catalyst Crystal size
/nm*ABET/
(m2·g-1)Pore volume
v/(cm3·g-1)dave
/nmFe2O3 40.2 12.5 0.058 18.2 FeAlZr1 28.6 59.5 0.225 10.9 FeAlZr2 26.5 141.3 0.246 5.0 FeAlCe1 30.2 42.2 0.208 13.9 FeAlCe2 30.4 54.8 0.189 9.8 *: the average crystal size: calculated by Scherrer formula according to the face (104) at 33° Table 3 Relative oxygen species percentage on co-doped and undoped Fe2O3 catalysts
Catalyst Oxygen species percentage /% O2- O- OH- O22- Fe2O3 75.8 7.3 5.8 11.1 FeAlZr1 73.8 18.5 4.9 2.8 FeAlZr2 71.6 12.7 8.4 7.3 FeAlCe1 69.5 9.4 13.4 7.7 FeAlCe2 63.9 10.0 15.5 10.6 Table 4 Water, pitch conversion and product distributions of coal tar over different co-doped Fe2O3 catalysts
Catalyst Conversion x/% Yield w/% water pitch light tar pitch residue coke gas Blank* - 24.8 47.7 26.2 21.8 - 4.3 Fe2O3 11.7 29.5 49.7 28.0 5.4 1.4 15.5 FeAlZr1 20.8 42.8 63.2 24.6 4.7 7.0 15.3 FeAlZr1no - 56.2 53.5 18.9 3.7 11.4 12.5 FeAlZr2 16.9 47.5 58.1 22.6 2.0 8.8 21.4 FeAlCe1 16.5 41.4 60.2 25.2 3.0 7.3 19.3 FeAlCe2 17.9 41.5 55.1 27.2 5.9 7.5 14.7 *: blank test: silica sand instead of catalyst was placed in the reactor;
no: no water additionTable 5 Gas composition in SCC of coal tar over iron containing mixed oxides
Catalyst Gas composition φ/% CO2 CH4 C2H4 C2H6 C3H8 H2 CO Blank* 4.0 33.6 23.9 13.3 9.7 9.2 6.3 Fe2O3 15.5 14.4 9.7 5.1 4.3 48.9 2.1 FeAlZr1 9.4 5.7 2.1 1.4 0.9 80.6 - FeAlZr1no 5.5 10.0 4.3 3.0 1.7 72.1 3.3 FeAlZr2 10.3 5.6 2.0 1.3 0.9 79.9 - FeAlCe1 11.9 5.5 2.1 1.3 0.8 78.5 - FeAlCe2 12.5 5.6 2.1 1.3 0.8 77.7 - *: blank test: silica sand instead of catalyst was placed in the reactor;
no: no water addition -
[1] SONOYAMA N, NOBUTA K, KIMURA T, HOSOKAI S, HAYASHI J, TAGO T, MASUDA T. Production of chemicals by cracking pyrolytic tar from Loy Yang coal over iron oxide catalysts in a steam atmosphere[J]. Fuel Process Technol, 2011, 92(4):771-775. doi: 10.1016/j.fuproc.2010.09.036 [2] SCHOBERT H H, SONG C. Chemicals and materials from coal in the 21st century[J]. Fuel, 2002, 81(1):15-32. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=5b41864118cc2025d12ad74de8c7e54d [3] ZHANG C, WU R C, XU G W. Coal pyrolysis for high-quality tar in a fixed-bed pyrolizer enhanced with internals[J]. Energy Fuels, 2014, 28(1):236-244. doi: 10.1021/ef401546n [4] JIN L J, BAI X Y, YANG L, DONG C, HU H Q, LI X. In-situ catalytic upgrading of coal pyrolysis tar on carbon-based catalyst in a fixed-bed reactor[J]. Fuel Process Technol, 2016, 147:41-46. doi: 10.1016/j.fuproc.2015.12.028 [5] ZHOU Q, ZOU T, ZHONG M, ZHANG Y M, WU R C, GAO S Q, XU G W. Lignite upgrading by multi-stage fluidized bed pyrolysis[J]. Fuel Process Technol, 2013, 116:35-43. doi: 10.1016/j.fuproc.2013.04.022 [6] HAN L N, ZHANG R, BI J C. Upgrading of coal-tar pitch in supercritical water[J]. J Fuel Chem Technol, 2008, 36(1):1-5. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rlhxxb200801001 [7] KHALIL U, MURAZA O, KONDOH H, WATANABE G, NAKASAKA Y, AL-AMER A, MASUDA T. Production of lighter hydrocarbons by steam-assisted catalytic cracking of heavy oil over Silane-treated Beta Zeolite[J]. Energy Fuels, 2016, 30(2):1304-1309. doi: 10.1021/acs.energyfuels.5b02525 [8] KONDOH H, TANAKA K, NAKASAKA Y, TAGO T, MASUDA T. Catalytic cracking of heavy oil over TiO2-ZrO2 catalysts under superheated steam conditions[J]. Fuel, 2016, 167:288-294. doi: 10.1016/j.fuel.2015.11.075 [9] LEE H S, NGUYEN-HUY C, PHAM T T, SHIN E W. ZrO2-impregnated red mud as a novel catalyst for steam catalytic cracking of vacuum residue[J]. Fuel, 2016, 165:462-467. doi: 10.1016/j.fuel.2015.10.083 [10] KONDOH H, NAKASAKA Y, KITAGUCHI T, YOSHIKAWA T, TAGO T, MASUDA T. Upgrading of oil sand bitumen over an iron oxide catalyst using sub-and super-critical water[J]. Fuel Process Technol, 2016, 145:96-101. doi: 10.1016/j.fuproc.2016.01.030 [11] GONG X M, WANG Z, LI S G, SONG W L, LIN W G. Coal pyrolysis in a laboratory-scale two-stage reactor:Catalytic upgrading of pyrolytic vapors[J]. Chem Eng Technol, 2014, 37(12):2135-2142. doi: 10.1002/ceat.201300748 [12] FUNAI S, FUMOTO E, TAGO T, MASUDA T. Recovery of useful lighter fuels from petroleum residual oil by oxidative cracking with steam using iron oxide catalyst[J]. Chem Eng Sci, 2010, 65(1):60-65. doi: 10.1016/j.ces.2009.03.028 [13] YAMAMOTO S, KENDELEWICZ T, NEWBERG J T, KETTELER G, STARR D E, MYSAK E R, ANDERSSON K J, OGASAWARA H, BLUHM H, SALMERON M, JR BROWN G E, NILSSON A. Water adsorption on α-Fe2O3 (0001) at near ambient conditions[J]. J Phys Chem C, 2010, 114:2256-2266. doi: 10.1021/jp909876t [14] FUMOTO E, MATSUMURA A, SATO S, TAKANOHASHI T. Recovery of lighter fuels by cracking heavy oil with zirconia-alumina-iron oxide catalysts in a steam atmosphere[J]. Energy Fuels, 2009, 23(1):1338-1341. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=a10d91aec5e76e0f2f4d73a316654d21 [15] HUANG L, TANG M C, FAN M H, FAN M H, CHEN H S. Density functional theory study on the reaction between hematite and methane during chemical looping process[J]. Appl Energy, 2015, 159:132-144. doi: 10.1016/j.apenergy.2015.08.118 [16] WANG T T, LI Y, JIN L J, WANG D C, HU H Q. Steam catalytic cracking of coal tar over iron-containing mixed metal oxides[J]. Can J Chem Eng, 2019, 97(3):702-708. doi: 10.1002/cjce.v97.3 [17] DONG C, JIN L J, LI Y, ZHOU Y, ZOU L, HU H Q. Integrated process of coal pyrolysis with steam reforming of methane for improving the tar yield[J]. Energy Fuels, 2014, 28(12):7377-7384. doi: 10.1021/ef501796a [18] WANG D C, JIN L J, LI Y, YAO D M, WANG J F, HU H Q. Upgrading of vacuum residue with chemical looping partial oxidation over Ce doped Fe2O3[J]. Energy, 2018, 162:542-553. doi: 10.1016/j.energy.2018.08.038 [19] NEWNHAM J, MANTRI K, AMIN M H, TARDIO J, BHARGAVA S K. Highly stable and active Ni-mesoporous alumina catalysts for dry reforming of methane[J]. Int J Hydrogen Energy, 2012, 37(2):1454-1464. doi: 10.1016/j.ijhydene.2011.10.036 [20] WANG D C, JIN L J, LI Y, HU H Q. Partial oxidation of vacuum residue over Al and Zr-doped α-Fe2O3 catalysts[J]. Fuel, 2017, 210:803-810. doi: 10.1016/j.fuel.2017.09.008 [21] STELMACHOWSKI P, KOPACZ A, LEGUTKO P, INDYKA P, WOJTASIK M, ZIEMIANSKI L, ZAK G, SOJKA Z, KOTARBA A. The role of crystallite size of iron oxide catalyst for soot combustion[J]. Catal Today, 2015, 257:111-116. doi: 10.1016/j.cattod.2015.02.018 [22] ZHU X, LI K Z, WEI Y G, WANG H, SUN L Y. Chemical-looping steam methane reforming over a CeO2-Fe2O3 oxygen carrier:Evolution of its structure and reducibility[J]. Energy Fuels, 2014, 28(2):754-760. doi: 10.1021/ef402203a [23] LIU Y, WEN C, GUO Y, LU G Z, WANG Y Q. Modulated CO oxidation activity of M-doped Ceria (M=Cu, Ti, Zr, and Tb):Role of the Pauling electronegativity of M[J]. J Phys Chem C, 2010, 114(21):9889-9897. doi: 10.1021/jp101939v [24] HAN X, YU Y B, HE H. Oxidative steam reforming of ethanol over Rh catalyst supported on Ce1-xLaxOy (x=0.3) solid solution prepared by urea co-precipitation method[J]. J Power Sources, 2013, 238:57-64. doi: 10.1016/j.jpowsour.2013.03.032 [25] TABATA K, KAWABE T, YAMAGUCHI Y, NAGASAWA Y. Chemisorbed oxygen species over the (110) face of SnO2[J]. Catal Surv Asia, 2003, 7(4):251-259. doi: 10.1023/B:CATS.0000008164.21582.92 [26] YAMAGUCHI Y, NAGASAWA Y, SHIMOMURA S, TABATA K, SUZUKI E. A density functional theory study of the interaction of oxygen with a reduced SnO2 (110) surface[J]. Chem Phys Lett, 2000, 316(5/6):477-482. http://www.sciencedirect.com/science/article/pii/S0009261499013652 [27] ARONNIEMI M, SAINIO J, LAHTINEN J. XPS study on the correlation between chemical state and oxygen-sensing properties of an iron oxide thin film[J]. Appl Surf Sci, 2007, 253(24):9476-9482. doi: 10.1016/j.apsusc.2007.06.007 [28] KAWABE T, SHIMOMURA S, KARASUDA T, TABATA K, SUZUKI E, YAMAGUCHI Y. Photoemission study of dissociatively adsorbed methane on a pre-oxidized SnO2 thin film[J]. Surf Sci, 2000, 448(2):101-107. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=f3f1533e6e073c9e5e1ac0ec3c7114ca [29] PURON H, ARRILLAGA P, CHIN K K, PINILLA J L, FIDALGO B, MILLA M. Kinetic analysis of vacuum residue hydrocracking in early reaction stages[J]. Fuel, 2014, 117:408-414. doi: 10.1016/j.fuel.2013.09.053 [30] CAPRARⅡS B, BRACCIALE M P, FILIPPIS P D, HERNANDEZ A D, PETRULLO A, SCARSELLA M. Steam reforming of tar model compounds over in supported on CeO2 and mayenite[J]. Can J Chem Eng, 2017, 95:1745-1751. doi: 10.1002/cjce.v95.9 [31] TOMISHIGE K, LI D L, TAMURA M, NAKAGAWA Y. Nickel-iron alloy catalysts for reforming of hydrocarbons:Preparation, structure, and catalytic properties[J]. Catal Sci Technol, 2017, 7(18):3952-3979. doi: 10.1039/C7CY01300K [32] HUY C N, SHIN E W. Amelioration of catalytic activity in steam catalytic cracking of vacuum residue with ZrO2-impregnated macro-mesoporous red mud[J]. Fuel, 2016, 179:17-24. doi: 10.1016/j.fuel.2016.03.062 -





 下载:
下载: