Study on release and transformation of iodine from anthracite during combustion
-
摘要: 采用逐级化学提取法研究无烟煤及其不同温度燃烧产物中碘的各种赋存状态;以小型管式炉模拟煤燃烧装置,考察了加热温度、加热时间、O2流量以及通入水蒸气对无烟煤中不同形态碘燃烧释放影响及其机理。结果表明,无烟煤中碘主要以有机结合态、铁锰氧化物结合态和水溶态形式存在。加热温度对碘释放和转化有明显影响,碘释放率随温度升高而增加,500-900℃是碘释放的主要阶段。其中,700℃以前,水溶态、离子交换态和有机结合态碘大部分释放,小部分转化为碳酸盐结合态、铁锰氧化物结合态和残留态碘;铁锰氧化物结合态碘主要在700-900℃释放,部分残留态碘在1 100℃前也可释放。无烟煤中碘释放率随燃烧时间延长和O2流量增大而增大,水蒸气的参与能明显促进碘的释放。在1 100℃、通入水蒸气、O2流量120 mL/min、燃烧20 min时,93.8%-95.9%碘主要以HI和I2释放。Abstract: Modes of iodine occurrence in 4 Chinese anthracites and the combustion residues obtained at each temperature were extracted with sequential chemical extraction. The coal combustion was simulated with a tube furnace, the effects and mechanisms of heating temperature, heating time, O2 flow rate and water vapor on iodine release and transformation behavior during anthracite combustion were investigated. The results showed iodine in anthracites can present typically in three main modes of occurrence:organic matter-bound, Fe-Mn oxide-bound and water-soluble form. Temperature had a pronounced effect on iodine release and transformation. Iodine release increased with the increase of temperature, and 500-900℃ was a main stage for iodine release. Before 700℃, some forms of iodine which include water-soluble, ion-exchangeable and organic matter-bound iodine can be almost emitted completely and partly transformed into the carbonate-bound, Fe-Mn oxide-bound and residue-bound iodine. The Fe-Mn oxide-bound iodine may be mainly emitted in 700-900℃, and the residue-bound iodine was emitted partly before 1 100℃.Moreover, the iodine release from anthracite increased with the increase of heating time and O2 flow rate, and water vapor can obviously promote the iodine release. 93.8%-95.9% of iodine may be emitted in the form of HI and I2 at the given experimental condition of 1 100℃, water vapor access, O2 flow rate of 120 mL/min and combustion time of 20 min.
-
Key words:
- anthracite /
- combustion /
- iodine /
- modes of occurrence /
- release /
- transformation
-
表 1 煤样的工业分析和元素分析
Table 1 Proximate and ultimate analyses of coal samples
Anthracite Proximate analysis wd/% Ultimate analysis wd/% M V FC A C H N O S A 5.00 5.52 75.02 15.46 78.21 3.02 1.06 6.44 0.39 B 5.06 5.37 70.36 19.21 72.34 2.05 1.19 4.83 0.21 C 5.71 6.29 70.21 17.79 79.66 2.61 1.41 5.54 0.27 D 7.85 6.80 66.45 18.84 73.28 2.48 1.50 5.76 0.51 表 2 高温灰成分分析
Table 2 High temperature ash analysis
Anthracite Content w/% MnO w/(mg·g-1) MgO Al2O3 SiO2 SO3 CaO Fe2O3 A 2.4 11.6 25.7 15.6 18.2 24.1 303.0 B 1.2 16.6 52.4 2.4 8.4 16.3 330.1 C 1.5 16.3 65.1 1.4 3.1 19.4 295.4 D 1.7 19.3 28.5 9.3 9.7 30.5 309.8 表 3 逐级化学提取无烟煤及其不同温度燃烧残渣中的碘
Table 3 Sequential chemical extraction of iodine in anthracite and combustion residue at each temperature
Step Methods Target phase 1 A 30 mL of H2O was added to 5.00 g coal or combustion residue, and the suspension was shaken for 24 h at 25 ℃. The leachate was separated by centrifugation at 3 500 r/min for 30 min and added to 50 mL with high-purity water, and then, the residue was subjected to the second extraction step water-soluble iodine 2 A 30 mL of 1.0 mol/L CH3COONH4 was added to the residue of step 1 The remaining methods were the same as step 1 lon-exchangeable iodine 3 A 30 mL of 1.0 mol/L CH3COONH4(pH 5.0 modified with CH3COONH4) was added to the residue of step 2 and the suspension was shaken for 24 h at 25 ℃. The leachate was subseque ntly separated by centrifugation, and the residue was washed with 10 mL of water The wash was combined with leachate and added to 50 mL with high-purity water, and then, the residue was dried at 95 ℃ for 30 h. After grinding and homogenization, a half of the residue was saved for the determination of iodine, and the other half was used for the next extraction step carbonate-bound iodine 4 A 15 mL solution containaing 0.04 mol/L NH2OH·HCl and 20%(v/v) CH3COOH was added to the half of the residue from step 3. The remaining methods were the same as step 3 Fe-Mn oxide-bound iodinea 5 A 4 mL of 0.02 mol/L HNO3 and a 4 mL of 30% H2O2 were added to the half of the residue from step 4, and the suspension was shaken for 2 h at 95 ℃, then separated with centrifugation. This operation was conducted twice. Then a 5 mL of 3.2 mol/L CHM3COONH4 and a 1 mL of 0.02 mol/L HNO3(pH 2.0) were added to the residue. The suspension was shake for 1 h at 25 ℃ before separation with centrifugation organic matter-bound iodinea 6 The residue from step 5 was dried at 95 ℃ for 30 h residue-bound iodine a: the differences of iodine value between the iodine in residues of step 3 and step 4, step 4 and step 5 were calculated as the iodine bound to the Fe-Mn oxide fraction and organic matter fraction, respectively 表 4 不同条件下无烟煤的燃烧
Table 4 Anthracite combustion under different conditions
Temperature t/℃ Time t/min O2 flow rate q/(mL·min-1) Water vapor X1 20 120 access 1 100 X2 120 access 1 100 20 X3 access 1 100 20 120 X4 X1: 300, 400, 500, 600, 700, 800, 900, 1 000, 1 100 ℃; X2: 5, 10, 15, 20, 25, 30, 35 min;
X3: 30, 60, 90, 120, 160, 200 mL/min; X4: water vapor access or not表 5 无烟煤中碘的含量及其赋存形态
Table 5 Iodine content in anthracite samples and their modes of occurrence by sequential extraction
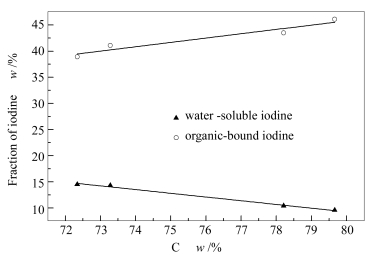
Anthracite Content w/(μg·g-1) E/(dry, %) E1 E2 E3 E4 E5 E6 A 8.3±0.6 10.4±1.1 0.00 0.00 39.2±1.2 43.5±1.5 6.91±0.4 B 8.9±0.3 14.5±1.0 0.00 7.06±0.5 29.0±0.9 38.9±2.0 3.87±0.3 C 9.3±0.2 9.56±0.8 0.42±0.2 0.00 37.9±1.3 46.1±1.9 6.12±0.3 D 5.3±0.4 14.3±1.2 8.06±0.7 0.00 32.4±1.2 41.1±1.7 5.11±0.4 E1, E2, E3, E4, E5 and E6 represent for the yield of extraction iodine of water-soluble, ion-exchangeable, carbonate-bound, Fe-Mn oxide-bound, organic matter-bound iodine and residue-bound iodine, respectively -
[1] IEA, World Energy Outlook, International Energy Agency, 2013. [2] XIN H H, WANG D M, QI X Y, QI G S, DOU G L. Structural characteristics of coal functional groups using quantum chemistry for quantification of infrared spectra[J]. Fuel Process Technol, 2014, 118:287-295. doi: 10.1016/j.fuproc.2013.09.011 [3] ZHOU H, ZHOU B, LI L, ZHANG H. Experimental measurement of the effective thermal conductivity of ash deposit for high sodium coal (Zhun Dong coal) in a 300 KW test furnace[J]. Energy Fuels, 2013, 27(11):7008-7022. doi: 10.1021/ef4012017 [4] FUGE R, JOHNSON C C. Iodine and human health, the role of environmental geochemistry and diet, a review[J]. Appl Geochem, 2015, 63:282-302. doi: 10.1016/j.apgeochem.2015.09.013 [5] STAGNARO-GREEN A, SULLIVAN S, PEARCE E N. Iodine supplementation during pregnancy and lactation[J]. JAMA, 2012, 308(23):2463-2464. doi: 10.1001/jama.2012.45423 [6] BETTINELLI M, SPEZIA S, MINOIA C, RONCHI A. Determination of chlorine, fluorine, bromine, and iodine in coals with ICP-MS and IC[J]. Atom Spectrosc, 2002, 23(4):105-110. [7] LUCY J C. Iodine in the marine boundary layer[J]. Chem Rev, 2003, 103(12):4953-4962. doi: 10.1021/cr0206465 [8] LANDSBERGER S, VERMETTE V G, WOLFE M, POWELL M A. Determination of halogens in coal using thermal and epithermal neutron activation analysis[J]. J Coal Qual, 1989, 8:95-97. [9] JAWOROWSKI Z, KOWNACKA L. Tropospheric and stratospheric distributions of radioactive iodine and cesium after the Chernobyl accident[J]. J Environ Radioact, 1988, 6(2):145-150. doi: 10.1016/0265-931X(88)90057-4 [10] WU D, DENG H, ZHENG B, WANG W, TANG X, XIAO H. Iodine in Chinese coals and its geochemistry during coalification[J]. Appl Geochem, 2008, 23(8):2082-2090. doi: 10.1016/j.apgeochem.2008.04.022 [11] 唐修义, 黄文辉.中国煤中微量元素[M].北京:商务印书馆, 2004:165.TANG Xiu-yi, HUANG Wen-hui. Trace Elements in Chinese Coal[M]. Beijing:Commercial Press, 2004:165. [12] WU D, DU J, DENG H, WANG W, XIAO H, LI P. Estimation of atmospheric iodine emission from coal combustion[J]. Int J Environ Sci Technol, 2014, 11:357-366. doi: 10.1007/s13762-013-0193-4 [13] MEIJ R, WINKEL TE H. The emissions of heavy metals and persistent organic pollutants from modern coal-fired power stations[J]. Atmos Environ, 2007, 41(40):9262-9272. doi: 10.1016/j.atmosenv.2007.04.042 [14] 高运川, 吴晓伟, 孙明星, 高勤芬, 刘勇弟.煤燃烧过程中痕量元素溴和碘的释放行为[J].华东理工大学学报, 2010, 36(4):482-487. http://www.cnki.com.cn/Article/CJFDTOTAL-HLDX201004005.htmGAO Yun-chuan, WU Xiao-wei, SUN Ming-xing, GAO Qin-fen, LIU Yong-di. Behavior of trace elements bromine and iodine during coal combustion process[J]. J East China Univer Sci Technol, 2010, 36(4):482-487. http://www.cnki.com.cn/Article/CJFDTOTAL-HLDX201004005.htm [15] PENG B X, LI L, WU D S. Distribution of bromine and iodine in thermal power plant[J]. J Coal Sci Eng, 2013, 19(3):387-391. doi: 10.1007/s12404-013-0320-3 [16] RATAFIA-BROWN J A. Overview of trace elements partitioning in flames and furnaces of utility coal-fired boilers[J]. Fuel Proces Technol, 1994, 39(2):139-157. doi: 10.1016/0378-3820(94)90177-5 [17] BLÄSING M, NAZERI K, MÜLLER M. Release of alkali metal, sulphur and chlorine species during high-temperature gasification and co-gasification of hard coal, refinery residue, and petroleum coke[J]. Fuel, 2014, 126:62-68. doi: 10.1016/j.fuel.2014.02.042 [18] VASSILEV S V, ESKENAZY G M, VASSILEVA C G. Contents, modes of occurrence and behaviour of chlorine and bromine in combustion wastes from coal-fired power stations[J]. Fuel, 2000, 79(8):923-937. doi: 10.1016/S0016-2361(99)00231-8 [19] IZQUIERDO M, QUEROL X. Leaching behaviour of elements from coal combustion fly ash:An overview[J]. Int J Coal Geol, 2012, 94:54-66. doi: 10.1016/j.coal.2011.10.006 [20] SIA S G, ABDULLAH W H. Enrichment of arsenic, lead, and antimony in Balingian coal from Sarawak, Malaysia:Modes of occurrence, origin, and partitioning behavior during coal combustion[J]. Int J Coal Geol, 2012, 101:1-15. doi: 10.1016/j.coal.2012.07.005 [21] 彭炳先, 吴代赦.烟煤和无烟煤中碘的赋存形态及其环境效应分析[J].燃料化学学报, 2012, 40(3):257-262. doi: 10.1016/S1872-5813(12)60013-9PENG Bing-xian, WU Dai-she. Modes of iodine occurrence in bituminous coal and anthracite and their environmental effects[J]. J Fuel Chem Technol, 2012, 40(3):257-262. doi: 10.1016/S1872-5813(12)60013-9 [22] WU D S, DENG H W, WANG W Y, XIAO H Y. Catalytic spectrophotometric determination of iodine in coal by pyrohydrolysis decomposition[J]. Anal Chim Acta, 2007, 601(2):183-188. doi: 10.1016/j.aca.2007.08.041 [23] SWAINE D J. Trace Elements in Coal[M]. Butterworth, London, 1990. [24] GUO R X, YANG J L, LIU D Y, LIU Z Y. Transformation behavior of trace elements during coal pyrolysis[J]. Fuel Process Technol, 2002, 77-78(20):137-143. https://www.researchgate.net/publication/257209166_Transformation_behavior_of_trace_elements_during_coal_pyrolysis -





 下载:
下载: