Determination of surface properties of direct coal liquefaction residue before and after solvent extraction by inverse gas chromatography
-
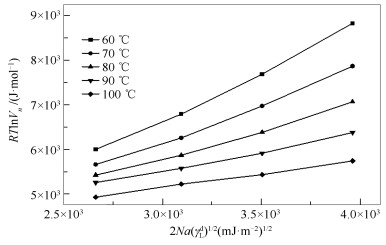
摘要: 采用反气相色谱法(IGC)分别对煤直接液化残渣(DCLR)脱灰处理后不溶物、正己烷不溶物、甲苯不溶物、四氢呋喃不溶物的表面性质进行表征。基于非极性探针净保留体积Vn分别采用Dorris-Gray方法和Schultz方法得到表面色散自由能,基于极性探针Vn得到吸附焓△Hsp,并通过△Hsp作图计算得到酸常数Ka和碱常数Kb。结果表明,经溶剂分级提取后表面色散自由能、Ka和Kb均发生明显变化。而DCLR呈现两性偏碱性这一特征并未随分级提取发生改变。IGC作为一种动力学吸附技术,可快速准确表征DCLR在经分级提取过程中表面性质的变化,相同温度下应用Dorris-Gray方法得到DCLR表面色散自由能略高于Schultz方法。Abstract: Direct coal liquefaction residue (DCLR) is deashed by hydrochloric acid and hydrofluoric acid to obtain ACLR. The ACLR was extracted with n-hexane, toluene and tetrahydrofuran to obtain n-hexane insoluble matter (HCLR), toluene insoluble matter (TCLR), tetrahydrofuran insoluble (FCLR). The surface properties of ACLR, HCLR, TCLR and FCLR were characterized by inverse gas chromatography (IGC). Based on net residual volume Vn of the non-polar probe, surface dispersion free energy were calculated by Dorris-Gray and Schultz method respectively. The adsorption enthalpy △Hsp of the polar probe on surface of the 4 insoluble solids was obtained by Vn of the polar probe, and the acid constant Ka and the base constant Kb of the 4 insoluble solids were calculated by △Hsp. The results show that the surface dispersion free energy of ACLR are obviously changed after solvent extraction, and the dispersion free energy on surface of the 4 insoluble solids is decreasing from 60 to 100℃. By Kb/Ka> 1 and △Hsp analysis, 4 insoluble solids surfaces are both amphoteric and the alkaline effect is stronger than that of the acid effect. As a kinetic adsorption technique, IGC can quickly and accurately characterize change of surface properties of DCLR during fractionated extraction. At the same temperature the surface dispersion free energy obtained by the Dorris-Gray method is slightly higher than that by the Schultz method.
-
表 1 伊犁煤的工业分析与元素分析
Table 1 Proximate and ultimate analyses of the Yili coal
Proximate analysis w/% Ultimate analysis wdaf /% Mad Ad Vdaf C H N S O 7.33 8.61 36.79 83.21 4.25 0.98 0.39 11.17 表 2 探针溶剂的性质
Table 2 Nature of the probe solvent
Probe a×1020/m2 γsd/(mJ·m-2) AN*/(kJ·mol-1) DN/(kJ·mol-1) n-C6 51.5 18.4 - - n-C7 57.0 20.3 - - n-C8 63.0 21.3 - - n-C9 69.0 22.7 - - CHCl3 44.0 25.9 22.7 0 Acet 42.5 16.5 10.5 71.4 Etacet 48.0 19.6 6.3 71.1 THF 45.0 22.5 2.1 84.4 表 3 分别采用Dorris-Gray(D-G)和Schultz(S)方法计算得到表面色散自由能
Table 3 Surface dispersion free energy calculated by Dorris-Gray and Schultz method
t/℃ ACLRγsd/(mJ·m-2) HCLRγsd/(mJ·m-2) TCLRγsd/(mJ·m-2) FCLRγsd/(mJ·m-2) D-G S D-G S D-G S D-G S 60 5.25 4.75 3.95 3.25 8.81 7.95 3.30 2.97 70 3.27 2.90 2.09 1.28 5.98 5.31 1.85 1.64 80 1.84 1.60 0.88 0.76 4.09 3.56 0.77 0.67 90 0.87 0.74 0.56 0.23 2.72 2.32 0.32 0.28 100 0.46 0.38 0.31 0.19 1.72 1.44 0.17 0.14 表 4 酸碱作用的吸附焓
Table 4 Adsorption enthalpy-△Hsp of acid-base interaction
Probe -△Hsp/(kJ·mol-1) ACLR HCLR TCLR FCLR CHCl3 11.49 13.44 11.60 13.49 Acet 12.55 16.22 9.99 11.63 Etacet 8.78 13.61 9.90 7.66 THF 9.16 11.61 7.47 5.33 表 5 固体不溶物表面酸碱常数
Table 5 Surface acid-base constant of solid insoluble
Kb Ka Kb/Ka R2 ACLR 0.458 0.096 4.750 0.994 HCLR 0.687 0.121 5.666 0.998 TCLR 0.543 0.076 7.165 0.988 FCLR 0.684 0.047 14.690 0.986 -
[1] KHARE S, DELL'AMICO M. An overview of solid-liquid separation of residues from coal liquefaction processes[J]. Can J Chem Eng, 2013, 91(2):324-331. doi: 10.1002/cjce.v91.2 [2] ZHOU Y, XIAO N, QIU J S, SUN Y F, SUN T J, ZHAO Z B, ZHANG Y, TSUBAKI N. Preparation of carbon microfibers from coal liquefaction residue[J]. Fuel, 2008, 87(15):3474-3476. http://cn.bing.com/academic/profile?id=8641171bf3e373841d007ce3a4475570&encoded=0&v=paper_preview&mkt=zh-cn [3] ZHANG J B, JIN L J, HE X F, LIU S B, HU H Q. Catalytic methane decomposition over activated carbons prepared from direct coal liquefaction residue by KOH activation with addition of SiO2 or SBA-15[J]. Int J Hydrogen Energy, 2011, 36(15):8978-8984. doi: 10.1016/j.ijhydene.2011.04.205 [4] ZHANG J B, JIN L J, ZHU S W, HU H Q. Preparation of mesoporous activated carbons from coal liquefaction residue for methane decomposition[J]. J Nat Gas Chem, 2012, 21(6):759-766. doi: 10.1016/S1003-9953(11)60429-5 [5] XIAO N, ZHOU Y, QIU J S, WANG Z H. Preparation of carbon nanofibers/carbon foam monolithic composite from coal liquefaction residue[J]. Fuel, 2010, 89(5):1169-1171. doi: 10.1016/j.fuel.2009.10.023 [6] XIAO N, ZHOU Y, LING Z, QIU J. Synthesis of a carbon nanofiber/carbon foam composite from coal liquefaction residue for the separation of oil and water[J]. Carbon, 2013, 59(4):530-536. http://cn.bing.com/academic/profile?id=8e3c6f59abab75fb891deb74e87c2cf0&encoded=0&v=paper_preview&mkt=zh-cn [7] LOZANO-CASTELLO D, LILLO-RODENAS M, CAZORLA-AMOR S D, LINARES-SOLANO A. Preparation of activated carbons from Spanish anthracite:Ⅰ. Activation by KOH[J]. Carbon, 2001, 39(5):751-759. doi: 10.1016/S0008-6223(00)00186-X [8] LEE S Y, RYU B H, HAN G Y, LEE T J, YOON K J. Catalytic characteristics of specialty carbon blacks in decomposition of methane for hydrogen production[J]. Carbon, 2008, 46(14):1978-1986. doi: 10.1016/j.carbon.2008.08.008 [9] SUELVES I, PINILLA J, L ZARO M, MOLINER R. Carbonaceous materials as catalysts for decomposition of methane[J]. Chem Eng J, 2008, 140(1):432-438. http://cn.bing.com/academic/profile?id=f7d6dc74052adc7a01921d10ce0c4996&encoded=0&v=paper_preview&mkt=zh-cn [10] XIE J, BOUSMINA M, XU G, KALIAGUINE S. Inverse gas chromatography studies of alkali cation exchanged X-zeolites[J]. J Mol Catal A:Chem, 1998, 135(2):187-197. doi: 10.1016/S1381-1169(97)00303-8 [11] THIELMANN F. Introduction into the characterisation of porous materials by inverse gas chromatography[J]. J Chromatogr A, 2004, 1037(1/2):115-123. http://cn.bing.com/academic/profile?id=c2f23b7561e34eb28b419401b8c48949&encoded=0&v=paper_preview&mkt=zh-cn [12] MUKHOPADHYAY P, SCHREIBER H P. Aspects of acid-base interactions and use of inverse gas chromatography[J]. Colloids Surf A, 1995, 100(95):47-71. http://cn.bing.com/academic/profile?id=1f0e872e39a26c6f234798a0f0d1fc0a&encoded=0&v=paper_preview&mkt=zh-cn [13] YOUNG, LESLIE C. Physicochemical Measurement by Gas Chromatography[M]. Hoboken:Wiley, 1979. [14] SHAN T H, DUDA J L. Probing coal structure with organic vapour sorption[J]. Fuel, 1987, 66(2):170-178. doi: 10.1016/0016-2361(87)90236-5 [15] THIELMANN F, BUTLER D A, WILLIAMS D R. Characterization of porous materials by finite concentration inverse gas chromatography[J]. Colloids Surf A, 2001, s187/188(6):267-272. http://cn.bing.com/academic/profile?id=6fa71b4dc0dc364717977e7bff5dcae3&encoded=0&v=paper_preview&mkt=zh-cn [16] RVCKRIEM M, ENKE D, HAHN T. Inverse gas chromatography (IGC) as a tool for an energetic characterisation of porous materials[J]. Microporous Mesoporous Mater, 2015, 209:99-104. doi: 10.1016/j.micromeso.2014.08.053 [17] GUHA O K, ROY J. Molecular probe chromatography of bituminous coal[J]. Fuel Process Technol, 1985, 11(2):113-125. doi: 10.1016/0378-3820(85)90022-0 [18] GUHA O K, ROY J. Molecular probe chromatography of selected lignites[J]. Fuel, 1985, 64(8):1164-1167. doi: 10.1016/0016-2361(85)90123-1 [19] GUHA O K, ROY J. Behavior of a high temperature heat treated bituminous coal as a gas chromatographic substrate[J]. Fuel Process Technol, 1988, 17(3):301-309. doi: 10.1016/0378-3820(88)90042-2 [20] GUHA O K, ROY J, CHOUDHURY A. Non-polar carbon adsorbents from coal:Effect of coal rank studied by molecular probe chromatography[J]. Fuel, 1991, 70(1):9-12. doi: 10.1016/0016-2361(91)90087-Q [21] GLASS A S, LARSEN J W. Surface thermodynamics for nonpolar adsorbates on Illinois No. 6 coal by inverse gas chromatography[J]. Energy Fuels, 1993, 7(6):994-1000. doi: 10.1021/ef00042a042 [22] GLASS A S, LARSEN J W. Coal surface properties. Specific and nonspecific interactions for polar molecules and surface tensions for hydrocarbons at the surface of illinois No. 6 coal[J]. Energy Fuels, 1994, 8(3):629-636. doi: 10.1021/ef00045a018 [23] AND A S G, STEVENSON D S. Surface thermodynamics for hydrocarbons on wyodak coals[J]. Energy Fuels, 1996, 10(3):797-805. doi: 10.1021/ef950224g [24] AND A S G, WENGER E K. Surface thermodynamics for polar adsorbates on wyodak coals[J]. Energy Fuels, 1998, 12(1):152-158. doi: 10.1021/ef970117h [25] 李文, 白进.煤的灰化学[M].北京:科学出版社, 2013.LI Wen, BAI Jin. Ash Chemistry of Coal[M]. Beijing:Science Press, 2013. [26] DE BOER J. The Dynamical Character of Adsorption Oxford University Press[M]. London:Oxford University Press, 1953. [27] LAVIELLE L. The role of the interface in carbon fibre-epoxy composites[J]. J Adhes, 1987, 23(1):45-60. doi: 10.1080/00218468708080469 [28] DORRIS G M, GRAY D G. Adsorption of n-alkanes at zero coverage on cellulose paper and wood fibers[J]. J Colloid Interface Sci, 1980, 77(2):353-362. doi: 10.1016/0021-9797(80)90304-5 [29] VOELKEL A, ANDRZEJEWSKA E, LIMANOWSKA-SHAW H, ANDRZEJEWSKI M. Acid-base surface properties of glass-ionomers determined by IGC[J]. Appl Surf Sci, 2005, 245(1):149-154. http://cn.bing.com/academic/profile?id=28cd05113afa5894c5d6dd37ad20b248&encoded=0&v=paper_preview&mkt=zh-cn [30] 何选明.煤化学[M].北京:冶金工业出版社, 2010.HE Xuan-ming. Coal Chemistry[M]. Beijing:Metallurgical Industry Press, 2010. [31] 刘振学, 魏贤勇, 周仕学, 王华兰.煤的溶剂萃取研究进展(Ⅰ)有机溶剂及其萃取机理[J].煤炭转化, 2003, 26(2):1-5. http://www.cnki.com.cn/Article/CJFDTotal-MTSD201506087.htmLIU Zhen-xue, WEI Xian-yong, ZHOU Shi-xue, WANG Hua-lan. Advancesing coal of solvent extraction studies part (Ⅰ) organic solvents and their extracting mechanism[J]. Coal Convers, 2003, 26(2):1-5. http://www.cnki.com.cn/Article/CJFDTotal-MTSD201506087.htm [32] 王晓华, 魏贤勇.煤的溶剂萃取研究进展[J].现代化工, 2003, 23(7):19-22. http://www.cnki.com.cn/Article/CJFDTOTAL-XDHG200307004.htmWANG Xiao-hua, WEI Xian-yong. Advances in coal solvent extraction[J]. Mod Chem Ind, 2003, 23(7):19-22. http://www.cnki.com.cn/Article/CJFDTOTAL-XDHG200307004.htm [33] BALARD H, BRENDLE E, PAPIRER E. Determination of the acid-base properties of solid surfaces using inverse gas chromatography:Advantages and limitations[M]. Boca Raton:CRC Press, 2000. [34] 陈茺, 李伟.煤中氢键类型的研究[J].燃料化学学报, 1998, 26(2):45-49. http://www.wanfangdata.com.cn/details/detail.do?_type=degree&id=Y788277CHEN Chong, LI Wei. Type of hydrogen bonds in coal[J]. J Fuel Chem Technol, 1998, 26(2):45-49. http://www.wanfangdata.com.cn/details/detail.do?_type=degree&id=Y788277 [35] WANG W, QIN Y, QIAN F, YE L, HAO W, YUAN L, JIN F. Partitioning of elements from coal by different solvents extraction[J]. Fuel, 2014, 125(2):73-80. http://cn.bing.com/academic/profile?id=ff72e9f435b9caebc1733dd2c68627a8&encoded=0&v=paper_preview&mkt=zh-cn -





 下载:
下载: