Evolution of nitrogen functionalities and their relation to NOx precursors during pyrolysis of antibiotic mycelia wastes
-
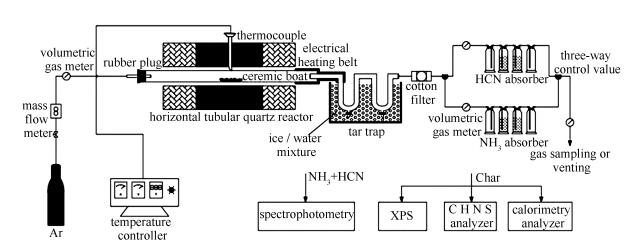
摘要: 以青霉素菌渣(PMW)和土霉素菌渣(TMW)为对象,在水平管式反应器中进行快速热解,采用X射线光电子能谱(XPS)表征和化学吸收-分光光度定量分析方法,研究了抗生素菌渣热解N官能团变化特征及其与NOx前驱物的关系。结果表明,菌渣燃料N官能团分为无机N(N-IN)和蛋白质及其水解产物N(N-A)两种。决定菌渣NOx前驱物以NH3-N为主,N官能团主要为N-A,PMW占81.1%、TMW占59.0%。在低温区间,N-IN在150-250℃分解和N-A在250-450℃转化,为NH3-N主要来源;PMW和TMW产率分别为20.9%和25.6%,而HCN-N产率小于2%,基本与燃料N官能团特征无关;该阶段伴随吡啶N(N-6)和吡咯N(N-5)的生成及转化,峰值在350-400℃。在高温区间,半焦N反应,主要是N-6和N-5的转化,为NH3-N和部分HCN-N的来源;该阶段伴随少量更稳定质子化吡啶N(N-Q)和氮氧化物N(N-X)生成。由于N-IN和不稳定N-A低温下会快速分解,250-300℃下菌渣半焦N去除高达40%、能量损失可控制在25%,因此,采用合适低温热解处理菌渣,在保证能量前提下可有效去除燃料中的N。Abstract: On the basis of rapid pyrolysis of two antibiotic mycelial wastes (AMWs), viz., penicillin mycelia waste (PMW) and terramycinmycelial waste (TMW), in a horizontal tubular quartz reactor, evolution of nitrogen functionalities and their relation to NOx precursors were investigated with the help of XPS and chemical absorption-spectrophotometry methods.The results indicate that inorganic-N (N-IN) and amide-N/amine-N/amino-N (N-A) are two kinds of nitrogen functionalities in the raw AMWs samples, determining the predominance of NH3-N among NOx precursors. N-A is found to be the main one with the proportion of 81.1% and 59.0% for PMW and TMW, respectively. At low temperatures, the decomposition of N-IN and the conversion of N-A mainly occur at 150-250℃ and 250-450℃, respectively, which are two routes for most NH3-N with yields of 20.9% (PMW) and 25.6% (TMW). While HCN-N is produced with a small amount less than 2%, having no relationship with the characteristics of nitrogen functionalities in fuels. Besides, pyridinic-N (N-6) and pyrrolic-N (N-5) are also formed and then converted with peak values at 350-400℃. At high temperatures, the conversion of N-6 and N-5 is prevailing, leading to the basically equal increments on NH3-N and HCN-N. Simultaneously, a minor amount of more stable quaternary nitrogen (N-Q) and N-oxide (N-X) is produced. Typically, due to the rapid decomposition of N-IN and labile N-A at low-temperature pyrolysis, nitrogen removal can reach up to 40% while energy loss can be controlled within 25% when pyrolyzing at 250-300℃. As a result, low-temperature pyrolysis could be an effective method for nitrogen removal whereas preserving the energy in AMWs.
-
Key words:
- AMWs /
- nitrogen functionalities /
- NOx precursors /
- low-temperature pyrolysis /
- nitrogen removal
-
表 1 抗生素菌渣的工业分析及元素分析
Table 1 Proximate and ultimate analyses of antibiotic mycelial wastes (AMWs)
AMW PMW TMW Proximate analysis wdb/% Ash 8.09 14.85 Volatile matter 78.51 73.90 Fixed carbon 13.40 11.25 HHV Q /(MJ·kg-1) 19.28 19.33 Ultimate analysis wdaf/% Carbon 48.07 50.60 Hydrogen 6.96 7.17 Nitrogen 8.04 10.93 Sulfur 0.57 0.81 Oxygen (by difference) 36.36 30.49 -
[1] 贡丽鹏, 郭斌, 任爱玲, 刘仁平, 宋汉宁.抗生素菌渣理化特性[J].河北科技大学学报, 2012, 33(2):190-196. doi: 10.7535/hbkd.2012yx02023GONG Li-peng, GUO Bin, REN Ai-ling, LIU Ren-ping, SONG Han-ning. Physical and chemical properties of antibiotics bacterial residue[J]. J Heibei Univ Sci Technol, 2012, 33(2):190-196. doi: 10.7535/hbkd.2012yx02023 [2] 许光文, 纪文峰, 刘周恩, 万印华, 张小勇.轻工生物质过程残渣高值化利用必要性与技术路线分析[J].过程工程学报, 2009, 9(3):618-624. http://www.cnki.com.cn/Article/CJFDTOTAL-HGYJ200903037.htmXU Guang-wen, JI Wen-feng, LIU Zhou-en, WAN Yin-hua, ZHANG Xiao-yong. Necessity and technical route of value-added utilization of biomass process residues in light industry[J]. Chin J Process Eng, 2009, 9(3):618-624. http://www.cnki.com.cn/Article/CJFDTOTAL-HGYJ200903037.htm [3] GUO B, GONG L, DUAN E, LIU R, REN A, HAN J, ZHAO W. Characteristics of penicillin bacterial residue[J]. J Air Waste Manage, 2012, 62(4):485-8. doi: 10.1080/10962247.2012.658956 [4] ZHANG G Y, MA D C, PENG C N, LIU X X, XU G W. Process characteristics of hydrothermal treatment of antibiotic residue for solid biofuel[J]. Chem Eng J, 2014, 252(252):230-238. https://www.cabdirect.org/cabdirect/abstract/20143295251 [5] Ma D C, Zhang G Y, Zhao P T, AREEPRASERT C, SHEN Y F, YOSHIKAWA K, XU G W. Hydrothermal treatment of antibiotic mycelial dreg:More understanding from fuel characteristics[J]. Chem Eng J, 2015, 273(8):147-155. http://www.irgrid.ac.cn/handle/1471x/975435 [6] 尤占平, 郝长生, 焦永刚, 赵亮, 封春红.两种抗生素菌渣热解及燃烧特性对比研究[J].工业安全与环保, 2016, 42(05):41-43. doi: 10.3969/j.issn.1001-425X.2016.05.012YOU Zhan-ping, HAO Chang-sheng, JIAO Yong-gang, ZHAO Liang, FENG Chun-hong. Pyrolysis and combustion characteristics comparison studies of two kinds of antibiotic residues[J]. Ind Safety Environ Prot, 2016, 42(05):41-43. doi: 10.3969/j.issn.1001-425X.2016.05.012 [7] 贡丽鹏. 土霉素菌渣热解技术的研究[D]. 石家庄: 河北科技大学, 2012.GONG Li-peng. Research on pyrolysis technology of terramycin bacterial residue[D]. Shijiazhuang:Hebei University of Science & Technology, 2012. [8] ZHOU B H, GAO Q, WANG H H, DUAN E H, GUO B, ZHU N. Preparation, characterization, and phenol adsorption of activated carbons from oxytetracycline bacterial residue[J]. J Air Waste Manage, 2012, 62(12):1394-1402. doi: 10.1080/10962247.2012.716013 [9] YANG S J, ZHU X D, WANG J S, XING J, LIU Y C, FENG Q, ZHANG S C, CHEN J M. Combustion of hazardous biological waste derived from the fermentation of antibiotics using TG-FTIR and Py-GC/MS techniques[J]. Bioresource Technol, 2015, 193:156-163. doi: 10.1016/j.biortech.2015.06.083 [10] DU Y Y, JIANG X G, MA X J, LIU X D, LÜ G J, JIN Y Q, WANG F, CHI Y, YAN J H. Evaluation of cofiring bioferment residue with coal at different proportions:Combustion characteristics and kinetics[J]. Energy Fuels, 2013, 27(10):6295-6303. doi: 10.1021/ef401536b [11] BALAT M, BALAT M, KIRTAY E, BALAT H. Main routes for the thermo-conversion of biomass into fuels and chemicals. Part 1:Pyrolysis systems[J]. Energy Convers Manage, 2009, 50(12):3147-3157. doi: 10.1016/j.enconman.2009.08.014 [12] HANSSON K M, SAMUELSSON J, TULLIN C, AMAND L E. Formation of HNCO, HCN, and NH3 from the pyrolysis of bark and nitrogen-containing model compounds[J]. Combust Flame, 2004, 137(3):265-277. doi: 10.1016/j.combustflame.2004.01.005 [13] CAO J J, SHEN Z X, CHOW J C, WATSON J G, LEE S C, TIE X X, HO K F, WANG G H, HAN Y M. Winter and summer PM2.5 chemical compositions in fourteen chinese cities[J]. J Air Waste Manage, 2012, 62(10):1214-1226. doi: 10.1080/10962247.2012.701193 [14] TIAN F J, LI B Q, CHEN Y, LI C Z. Formation of NOx precursors during the pyrolysis of coal and biomass. Part Ⅴ. Pyrolysis of a sewage sludge[J]. Fuel, 2002, 81(17):2203-2208. doi: 10.1016/S0016-2361(02)00139-4 [15] TIAN F J, YU J L, MCKENZIE L J, HAYASHI J I, CHIBA T, LI C Z. Formation of NOx precursors during the pyrolysis of coal and biomass. Part Ⅶ. Pyrolysis and gasification of cane trash with steam[J]. Fuel, 2005, 84(4):371-376. doi: 10.1016/j.fuel.2004.09.018 [16] CAO J P, LI L Y, MORISHITA K, XIAO X B, ZHAO X Y, WEI X Y, TAKARADA T. Nitrogen transformations during fast pyrolysis of sewage sludge[J]. Fuel, 2013, 104:1-6. doi: 10.1016/j.fuel.2010.08.015 [17] TIAN Y, ZHANG J, ZUO W, CHEN L, CUI Y N, TAN T. Nitrogen conversion in relation to NH3 and HCN during microwave pyrolysis of sewage sludge[J]. Environ Sci Technol, 2013, 47(7):3498-3505. doi: 10.1021/es304248j [18] WEI L H, WEN L, YANG T H, ZHANG N. Nitrogen transformation during sewage sludge pyrolysis[J]. Energy Fuels, 2015, 29(8):5088-5094. doi: 10.1021/acs.energyfuels.5b00792 [19] 成建华, 张文莉.抗生素菌渣处理工艺设计[J].化工与医药工程, 2003, 24(2):31-34. http://www.cnki.com.cn/Article/CJFDTOTAL-YGCJ200302014.htmCHENG J H, ZHANG W L. Technological design of antibiotic residue treatment[J]. Chem Pharm Eng, 2003, 24(2):31-34. http://www.cnki.com.cn/Article/CJFDTOTAL-YGCJ200302014.htm [20] MA D C, ZHANG G Y, AREEPRASERT C, LI C X, SHEN Y F, YOSHIKAWA K, XU G W. Characterization of NO emission in combustion of hydrothermally treated antibiotic mycelial residue[J]. Chem Eng J, 2016, 284(1):708-715. https://www.deepdyve.com/lp/elsevier/characterization-of-no-emission-in-combustion-of-hydrothermally-5KziAkwD9K [21] ZHU X D, YANG S J, WANG L, LIU Y C, QIAN F, YAO W Q, ZHANG S C, CHEN J M. Tracking the conversion of nitrogen during pyrolysis of antibiotic mycelial fermentation residues using XPS and TG-FTIR-MS technology[J]. Environ Pollut, 2016, 211:20-27. doi: 10.1016/j.envpol.2015.12.032 [22] CHEN H F, WANG Y, XU G W, YOSHIKAWA K. Fuel-N evolution during the pyrolysis of industrial biomass wastes with high nitrogen content[J]. Energies, 2012, 5(12):5418-5438. doi: 10.3390/en5125418 [23] ZHAN H, YIN X L, Huang Y Q, Zhang X H, Yuan H Y, Xie J J, Wu C Z. Characteristics of NOx precursors and their formation mechanism during pyrolysis of herb residues[J]. J Fuel Chem Technol, 2017, 45(3):279-288. doi: 10.1016/S1872-5813(17)30017-8 [24] KELEMEN S R, AFEWORKI M, GORBATY M L, KWIATEK P J, SANSONE M, WALTERS C C, COHEN A D. Thermal transformations of nitrogen and sulfur forms in peat related to coalification[J]. Energy Fuels, 2006, 20(2):635-652. doi: 10.1021/ef050307p [25] TIAN K, LIU W J, QIAN T T, JIANG H, YU H Q. Investigation on the evolution of N-containing organic compounds during pyrolysis of sewage sludge[J]. Environ Sci Technol, 2014, 48(18):10888-10896. doi: 10.1021/es5022137 [26] 李梅, 杨俊和, 张启锋, 常海洲, 孙慧.用XPS研究新西兰高硫煤热解过程中氮、硫官能团的转变规律[J].燃料化学学报, 2013, 41(11):1287-1293. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18287.shtmlLI Mei, YANG Jun-he, ZHANG Qi-feng, CHANG Hai-zhou, SUN Hui. XPS study on transformation of N-and S-functionalgroups during pyrolysis of high sulfur New Zealand coal[J]. J Fuel Chem Technol, 2013, 41(11):1287-1293. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18287.shtml [27] 郭斌, 贡丽鹏, 刘仁平, 任爱玲, 宋汉宁.土霉素菌渣的热解特性及动力学研究[J].太阳能学报, 2013, 34(9):1504-1508. http://www.cnki.com.cn/Article/CJFDTOTAL-TYLX201309003.htmGUO Bin, GONG Li-peng, LIU Ren-ping, REN Ai-ling, SONG Han-ling. Study on pyrolysis characteristics and kinetics of terramycin bacterial residue[J]. Acta Energi Sin, 2013, 34(9):1504-1508. http://www.cnki.com.cn/Article/CJFDTOTAL-TYLX201309003.htm [28] 詹昊, 张晓鸿, 阴秀丽, 吴创之.生物质热化学转化过程含N污染物形成研究进展[J].化学进展, 2016, 28(12):1880-1890. doi: 10.7536/PC160438ZHAN Hao, ZHANG Xiao-hong, YIN Xiu-li, WU Chuang-zhi. Formation of nitrogenous pollutants during biomass thermo-chemical conversion[J]. Prog Chem, 2016, 28(12):1880-1890. doi: 10.7536/PC160438 [29] CHEN H F, NAMIOKA T, YOSHIKAWA K. Characteristics of tar, NOx precursors and their absorption performance with different scrubbing solvents during the pyrolysis of sewage sludge[J]. Appl Energ, 2011, 88(12):5032-5041. doi: 10.1016/j.apenergy.2011.07.007 [30] HANSSON K M, AMAND L E, HABERMANN A, WINTER F. Pyrolysis of poly-L-leucine under combustion-like conditions[J]. Fuel, 2003, 82(6):653-660. doi: 10.1016/S0016-2361(02)00357-5 [31] 刘海明, 张军营, 郑楚光, 孟韵.煤中吡咯型和吡啶型氮热解稳定性研究[J].华中科技大学学报:自然科学版, 2004, 32(11):13-15. http://www.cnki.com.cn/Article/CJFDTOTAL-HZLG200411004.htmLIU Hai-ming, ZHANG Jun-ying, ZHENG Chu-guang, MENG Yun. Quantum chemical study of the pyrolysis stability of pyrrolic nitrogen and pyridinic nitrogen in coal[J]. J Huazhong Univ Sci Technol:Nat Sci Ed, 2004, 32(11):13-15. http://www.cnki.com.cn/Article/CJFDTOTAL-HZLG200411004.htm [32] XIE Z L, FENG J, ZHAO W, XIE K C, PRATT K C, LI C Z. Formation of NOx and SOx precursors during the pyrolysis of coal and biomass. Part Ⅳ. Pyrolysis of a set of Australian and Chinese coals[J]. Fuel, 2001, 80(15):2131-2138. doi: 10.1016/S0016-2361(01)00103-X -





 下载:
下载: