Enhancement of thermal oxidation stability of endothermic hydrocarbon fuels by using oxygen scavengers
-
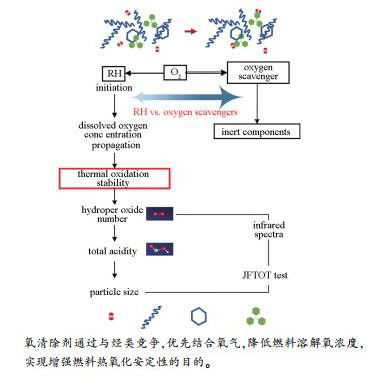
摘要: 热氧化安定性是吸热型碳氢燃料贮存和使用过程中评价燃料品质的重要性质之一,反映了喷气燃料在260℃以下组分受溶解氧影响的程度和燃料氧化反应进行的深度。为评价氧清除剂,选用一种实验室自制的吸热型燃料,运用加速氧化法,配合滴定、红外光谱、粒度分布和JFTOT等测试方法对燃料的基础物性和热氧化安定性进行评估,比较了三苯基膦(TPP)、二环己基苯基膦(DCP)和1,2,5-三甲基吡咯(TMP)三种氧清除剂对吸热型碳氢燃料自氧化过程的影响,并确定了测试范围内的最佳添加量。结果显示,三种氧清除剂的添加对燃料的组成和基础物性无明显影响;燃料中的溶解氧浓度随添加量增加不断下降,最大可降低溶氧浓度31.95 mg/m3;加速氧化后,样品的过氧化值和酸值均不同程度下降;胶团粒径分布趋向于更小粒径方向;JFTOT测试结果均满足国标规定。总体上,氧清除剂的添加均能有效提升燃料的热氧化安定性,三者的最优添加量均为质量分数1.5×10-5,作用效果优劣顺序为TMP>TPP≈DCP。Abstract: Thermal oxidation stability is one of the important properties for evaluating fuel quality during the storage and use of endothermic hydrocarbon fuels; it reflects the extent to which jet fuel is affected by dissolved oxygen below 260℃ and the depth of oxidation reaction. In this work, the basic properties and thermal stability of endothermic hydrocarbon fuels with oxygen scavengers were evaluated by accelerated oxidation method combined with titration, infrared spectroscopy, particle size distribution and JFTOT. The effect of three oxygen scavengers, viz., triphenylphosphine (TPP), dicyclohexylphenylphosphine (DCP) and 1, 2, 5-trimethylpyrrole (TMP), on the auto-oxidation process of endothermic hydrocarbon fuels were comparatively investigated and the optimal addition amount within the test range was determined. The results show that there is no significant change in the fuel composition and basic physical properties after the addition of oxygen scavengers. The dissolved oxygen concentration in the fuel decreases with the increase of the amount of oxygen scavengers, and the maximum drop is 31.95 mg/m3. The peroxide value and acid value of the samples show different degrees of decline after accelerated oxidation, and the particle size distribution of the micelles tends to be smaller. The JFTOT test results can meet the national standards. In general, the addition of oxygen scavenger can effectively improve the thermal oxidation stability of the fuel; the effect of three oxygen scavengers follows the order of TMP >TPP ≈ DCP and the optimal addition amount is 1.5×10-5 (by mass fraction).
-
表 1 EHF-2燃料基础物性表
Table 1 Specification properties of EHF-2
Property Test result Test standards Density(20 ℃) /(g·cm-3) 0.8505 GB/T-1884—2000[17] Heating value Q/(MJ·kg-1) 45.81 GB/T-384—1981[18], GB/T-2429—1988[19] Flash point t/℃ 64 GB/T-3536—2008[20] Viscosity(20 ℃) v/(mm2·s-1) 2.5763 GB/T-265—1988[21] Hydroperoxide number /×10-6 0 GB/T-601—2016[22], SH/T-0176—1992[23] Total acidity /(mgKOH·g-1) 0 GB/T-601—2016[22], GB/T-12574—1990[24] JFTOT test passed GB/T-9169—2010[25] Saybolt color +30 GB/T-3555—1992[26] Doctor test passed SH/T-0174—2015[27] 表 2 添加不同浓度氧清除剂的燃料基础物性
Table 2 Physical properties of EHF-2 with different concentrations of oxygen scavengers
w/×10-5 TPP DCP TMP viscosity at 20 ℃ v/(mm2·s-1) heat value Q/(MJ·kg-1) viscosity at 20 ℃ v/(mm2·s-1) heat value Q/(MJ·kg-1) viscosity at 20 ℃ v/(mm2·s-1) heat value Q/(MJ·kg-1) 0 2.5763 45.81 2.5763 45.81 2.5763 45.81 1.0 2.5748 45.90 2.5755 45.89 2.5758 45.86 2.5 2.5754 45.89 2.5761 45.89 2.5761 45.89 3.5 2.5755 45.87 2.5756 45.90 2.5752 45.89 4.5 2.5763 45.90 2.5753 45.89 2.5763 45.89 表 3 氧清除剂最优添加量下样品JFTOT测试结果
Table 3 JFTOT test of fuel samples under the optimal oxygen scavenger dosage
Sample Tube deposit rating (TDR) Mercury column heighta /mm Evaluation EHF-2 < 3 0.8 qualified DCP-15 < 1 0.2 qualified TPP-15 < 1 0.1 qualified TMP-15 < 1 0.1 qualified note: a: in the JFTOT test, the maximum pressure difference of the system is reflected by the mercury column height, which should not exceed 25 mm -
[1] 巴波可K K (苏).喷气燃料与火箭燃料[M].张溥等译.北京: 中国工业出版社, 1965.BABOK K K. Jet Fuel and Rocket Fuel[M]. ZHANG Pu et al, translation. Beijing: China Industry Press, 1965. [2] TAYLOR W F. Jet Fuel Thermal Stability[Z]. USA: NASA, 1979. [3] 杰尼索夫E T, 柯瓦列夫Г И (苏).喷气燃料的氧化及其抑制[M].常汝楫译.北京: 烃加工出版社, 1987.DENNSOV E T, KOVALEV G I. Oxidation & Antioxidation of Jet Fuel[M]. CHANG Ru-ji translation. Beijing: Hydrocarbon Processing Press, 1987. [4] 范启明, 米镇涛, 张香文, 于燕.提高航空燃料热安定性的研究进展[J].石化技术与应用, 2002, 20(4):261-263. doi: 10.3969/j.issn.1009-0045.2002.04.014FAN Qi-ming, MI Zhen-tao, ZHANG Xiang-wen, YU Yan. Progress in research of improving thermal stability of aviation fuels[J]. Petrochem Technol Appl, 2002, 20(4):261-263. doi: 10.3969/j.issn.1009-0045.2002.04.014 [5] ROAN M A, BOEHMAN A L. The effect of fuel composition and dissolved oxygen on deposit formation from potential JP-900 basestocks[J]. Energy Fuels, 2004, 18(3):835-843. doi: 10.1021-ef034050b/ [6] ZABARNICK S. Chemical kinetic modeling of jet fuel autoxidation and antioxidant chemistry[J]. Ind Eng Chem Res, 1993, 32(6):1012-1017. doi: 10.1021/ie00018a003 [7] ALBORZI E, GADSBY P, ISMAIL M S, SHEIKHANSARI A, DWYER M R, MEIJER A J, BLAKEY S G, POURKASHANIAN M. Comparative study of the effect of fuel deoxygenation and polar species removal on jet fuel surface deposition[J]. Energy Fuels, 2019, 33(3):1825-1836. [8] 王晨臣, 彭孝天, 王苏明, 刘卫华, 冯诗愚.溶解氧含量对燃油结焦的影响综述[J].中国民航飞行学院学报, 2018, 29(5):19-22+27. doi: 10.3969/j.issn.1009-4288.2018.05.005WANG Chen-chen, PENG Xiao-tian, WANG Su-ming, LIU Wei-hua, FENG Shi-yu. Summarization of the effect of dissolved oxygen content on fuel coking[J]. J Civil Avia Flight Univ Chin, 2018, 29(5):19-22+27. doi: 10.3969/j.issn.1009-4288.2018.05.005 [9] BEAVER B D. Development of oxygen scavenger additives for jet fuels[D]. Pittsburgh: Duquesne University, 1993. [10] BEAVER B D, DEMUNSHI R, HENEGHAN S P, WHITACRE S D, NETA P. Model studies directed at the development of new thermal oxidative stability enhancing additives for future jet fuels[J]. Energy Fuels, 1997, 11(2):396-401. doi: 10.1021/ef960192c [11] BEAVER B D, GAO L, FEDAK M G, COLEMAN M M, SOBKOWIAK M. Model studies examining the use of dicyclohexylphenylphosphine to enhance the oxidative and thermal stability of future jet fuels[J]. Energy Fuels, 2002, 16(5):1134-1140. doi: 10.1021/ef020028r [12] BEAVER B D, TENG Y, GUIRIEC P, HAPIOT P, NETA P. Mechanisms of oxidation of 1, 2, 5-trimethylpyrrole:Kinetic, spectroscopic, and electrochemical studies[J]. J Phys Chem A, 1998, 102(30):6121-6128. http://openurl.ebscohost.com/linksvc/linking.aspx?stitle=Journal%20of%20Physical%20Chemistry%20A&volume=102&issue=30&spage=6121 [13] THAVASI V, BETTENS R P A, LEONG L P. Temperature and solvent effects on radical scavenging ability of phenols[J]. J Phys Chem A, 2009, 113(13):3068-3077. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=fdffdadb30c2a91fd78d51f064e6e4ae [14] MACLEAN P D, CHAPMAN E E, DOBROWOLSKI S L, THOMPSON A, BARCLAY L R C. Pyrroles as antioxidants:Solvent effects and the nature of the attacking radical on antioxidant activities and mechanisms of pyrroles, dipyrrinones, and bile pigments[J].J Org Chem, 2008, 73(17):6623-6635. doi: 10.1021/jo8005073 [15] IUGA C, URIBE S O, MIRANDA L D, VIVIER BUNGE A. Selectivity in radical alkylation of substituted pyrroles[J]. Int J Quantum Chem, 2010, 110(3):697-705. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=ac1ed205e1deffc0c0b7cb7f064d3cd4 [16] GB/T-6537-2018, 3号喷气燃料[S].GB/T-6537-2018, No.3 jet fuel[S]. [17] GB/T-1884-2000, 原油和液体石油产品密度实验室测定法(密度计法)[S].GB/T-1884-2000, Crude petroleum and liquid petroleum products-Laboratory determination of density-Hydrometer method[S]. [18] GB/T-384-1981, 石油产品热值测定法[S].GB/T-384-1981, Determination of calorific value of petroleum products[S]. [19] GB/T-2429-1988, 航空燃料净热值计算法[S].GB/T-2429-1988, Aviation fuels-Calculation of net heat of combustion[S]. [20] GB/T-3536-2008, 石油产品闪点和燃点的测定-克利夫兰开口杯法[S].GB/T-3536-2008, Petroleum products-Determination of flash and fire points-Cleveland open cup method[S]. [21] GB/T-265-1988, 石油产品运动粘度测定法和动力粘度计算法[S].GB/T-265-1988, Petroleum products-Determination of kinematic viscosity and calculation of dynamic viscosity[S]. [22] GB/T 601-2016, 化学试剂标准滴定溶液的制备[S].GB/T 601-2016, Chemical reagent-Preparations of reference titration solutions[S]. [23] SH/T 0176-1992, 喷气燃料过氧化值测定法[S].SH/T 0176-1992, Standard test method for hydroperoxide number of aviation turbine fuels, gasoline and diesel fuels[S]. [24] GB/T 12574—1990, 喷气燃料总酸值测定法[S].GB/T 12574—1990, Jet fuels-Determination of total acid number[S]. [25] GB/T-9169-2010, 喷气燃料热氧化安定性的测定JFTOT法[S].GB/T-9169-2010, Standard test method for thermal oxidation stability of aviation turbine fuels.JFTOT procedure[S]. [26] GB/T-3555-1992, 石油产品塞伯特颜色测试法[S].GB/T-3555-1992, Petroleum products-Determination of Saybolt color-Saybolt chromometer method[S]. [27] SH/T-0174-2015, 石油产品和烃类溶剂中硫醇和其他硫化物的检测博士实验[S].SH/T-0174-2015, Petroleum products and hydrocarbon solvents-Detection of thiols and other sulfur species-Doctor test[S]. [28] ZHAO L, ZHANG X, PAN L, LIU J. Storage period prediction and metal compatibility of endothermic hydrocarbon fuels[J]. Fuel, 2018, 233:1-9. doi: 10.1016/j.fuel.2018.06.034 [29] MIL-T-5624P, Military specification: Turbine fuel, aviation, grades JP-4, JP-5, and JP-5 / JP-8[S]. [30] ERVIN J, HENEGHAN S, MARTEL C, WILLIAMS T. Surface effects on deposits from jet fuels[J]. J Eng Gas Turb Power, 1996, 118(2):278-285. doi: 10.1115/1.2816589 [31] BEAVER B D, CLIFFORD C B, FEDAK M G, GAO L, IYER P S, SOBKOWIAK M. High heat sink jet fuels. Part 1. Development of potential oxidative and pyrolytic additives for JP-8[J]. Energy Fuels, 2006, 20(4):1639-1646. doi: 10.1021/ef050352x [32] MASNOVI J, KOCHI J. Direct observation of ion-pair dynamics[J]. J Am Chem Soc, 1985, 107(26): 7880-7893. -





 下载:
下载: