Various basic nitrogen compounds removal from model diesel by adsorption with allochroic silica gel
-
摘要: 研究了变色硅胶吸附脱除氮含量为960.56 μg/g模拟柴油中的碱性氮化物喹啉、苯胺和吡啶。比较了氧化铝、硅藻土、硅胶及变色硅胶对模拟柴油中喹啉的吸附脱除效果。采用XRD、低温N2吸附-脱附和NH3-TPD等方法对硅胶和变色硅胶进行了表征。考察了粒径、吸附温度、吸附时间、剂油质量比及共存芳香化合物(萘、苯或甲苯)对变色硅胶吸附脱除各种碱性氮化物的影响。变色硅胶吸附脱除碱性氮化物的顺序均为苯胺>吡啶>喹啉。吸附时间对三种氮化物的吸附脱除没有影响;吸附温度、变色硅胶粒径和共存芳香化合物对苯胺和吡啶的吸附脱除效果影响不大,对喹啉的吸附脱除效果影响较为明显;剂油质量比对三种氮化物的吸附脱除影响均较大,尤其是对喹啉影响最大。结果表明,变色硅胶吸附各种氮化物时Co能够与其中的N原子形成配位络合吸附。经焙烧再生,变色硅胶几乎完全恢复了对喹啉和吡啶的吸附脱除能力,并可多次再生,但变色硅胶再生后对苯胺的吸附能力损失较大。Abstract: The allochroic silica gel was used for adsorptive denitrification from model diesel containing known amounts of quinoline, aniline or pyridine with a total nitrogen concentration 960.56 μg/g. The adsorptive removal of quinoline in model diesel with alumina, diatomite, silica gel and allochroic silica gel was investigated. The experiment results indicate that the adsorptive denitrification performance of allochroic silica gel is more superior to that of other three adsorbents, implying that the CoCl2 in allochroic silica gel can significantly improve the performance of denitrification. The silica gel and allochroic silica gel were characterized with X-ray diffraction (XRD), nitrogen adsorption and NH3-TPD. The XRD results indicate that the two samples are of an amorphous structure. Silica gel and allochroic silica gel have the average pore diameter of 18.46 and 1.80 nm, the Brunauer-Emmett-Teller (BET) surface area of 437.86 and 623.39 m2/g, and the pore volume of 0.9724 and 0.3442 m3/g, respectively. The results of NH3-TPD show that the acidity of allochroic silica gel is much stronger than that of silica gel which greatly enhances the adsorptive denitrification. Also, the influence of particle size, adsorption temperature, adsorption time, adsorbent to oil mass ratio and aromatic compounds on the adsorptive denitrification of allochroic silica gel was investigated. The adsorptive denitrification for different model diesels by allochroic silica gel is ordered as:aniline > pyridine > quinoline. Adsorption time has almost no influence on the removal of three nitrogen compounds. Adsorption temperature, particle size and aromatic compounds in the model diesel have little impact on the removal of aniline and pyridine, but have evident influences on the removal of quinoline. The adsorbent to oil ratio has a significant effect on the adsorptive denitrification, especially for quinoline. The experimental results suggest that the N-Co bond between Co in allochroic silica gel and N atom in the nitrogen compounds plays a significant role. Furthermore, the allochroic silica gel could be easily regenerated to recover its adsorptive denitrification for quinoline and pyridine by calcination once or several times, but except aniline.
-
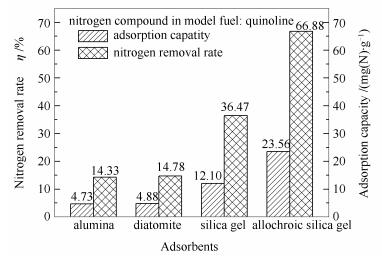
图 1 不同吸附剂吸附脱除模拟柴油中喹啉的脱氮率和吸附容量
Figure 1 Nitrogen removal rate and adsorption capacity for quinoline in model fuel with different adsorbents experimental conditions: room temperature; adsorbents:diatomite and 140-160 mesh alumina, silica gel or allochroic silica gel; the adsorbent to oil mass ratio=1:35; adsorption time 0.5 h
-
[1] 李振光, 袁建国.中国柴油需求趋势分析[J].国际石油经济, 2015, 23(9):88-93. http://www.docin.com/p-1433478269.htmlLI Zheng-guang, YUAN Jian-guo. China's diesel demand[J]. Int Pet Econ, 2015, 23(9):88-93. http://www.docin.com/p-1433478269.html [2] 杨昆昊, 夏赞宇, 何芃, 吴丽, 龚玲玲, 钱越英, 侯琰霖, 何裕建.机动车燃油质量及尾气排放与北京市大气污染的相关性[J].中国科学院大学学报, 2017, 34(3):305-317. https://www.cnki.com.cn/qikan-SYSA200405003.htmlYANG Kun-hao, XIA Zan-yu, HE Peng, WU Li, GONG Ling-ling, QIAN Yue-ying, HOU Yan-lin, HE Yu-juan. Correlation of fuel quality and emissions of motor vehicle with atmospheric pollution in Beijing[J]. J Univ Chin Acad Sci, 2017, 34(3):305-317. https://www.cnki.com.cn/qikan-SYSA200405003.html [3] LI N, ALMARRI M, MA X L, ZHA Q F. The role of surface oxygen-containing functional groups in liquid-phase adsorptive denitrogenation by activated carbon[J]. New Carbon Mater, 2011, 26(6):470-478. doi: 10.1016/S1872-5805(11)60093-0 [4] 文婕, 孙文晶, 杨文.氧化改性活性炭吸附脱氮选择性研究[J].功能材料, 2013, 20(40):2954-2958. http://manu50.magtech.com.cn/Jwk_gncl/CN/Y2013/V44/I20/2954WEN Jie, SUN Wen-jing, YANG Wen. Effects of oxidative modification of carbon surface on selective removal of nitrogen compounds from model fuel[J]. J Funct Mater, 2013, 20(40):2954-2958. http://manu50.magtech.com.cn/Jwk_gncl/CN/Y2013/V44/I20/2954 [5] 李红跃, 王雷, 张曼, 刘宝玉, 王立新, 刘丹.负载型杂多酸脱除焦化蜡油中碱性氮化物[J].化工进展, 2016, 35(3):826-830. http://d.old.wanfangdata.com.cn/Periodical/hgjz201603029LI Hong-yue, WANG Lei, ZHANG Man, LIU Bao-yu, WANG Li-xin, LIU Dan. Removal of basic nitrogen compounds in coker gas oil by supported heteropoly acid[J]. Chem Ind Eng Prog, 2016, 35(3):826-830. http://d.old.wanfangdata.com.cn/Periodical/hgjz201603029 [6] SEO P W, AHMED I, JHUNG S H. Adsorptive removal of nitrogen containing compounds from a model fuel using a metal organic framework having a free carboxylic acid group[J]. Chem Eng J, 2016, 299:236-243. doi: 10.1016/j.cej.2016.04.060 [7] KIM J H, MA X L, ZHOU A N. Ultra-deep desulfurization and denitrogenation of diesel fuel by selective adsorption over three different adsorbents:A study on adsorptive selectivity and mechanism[J]. Catal Today, 2006, 111:74-83. doi: 10.1016/j.cattod.2005.10.017 [8] LI C, SHEN B X, LIU J C. The removal of organic nitrogen compounds in naphtha by adsorption[J]. Energ Source Part A, 2013, 35:2348-2355. doi: 10.1080/15567036.2010.535098 [9] 徐晓宇, 孙悦, 沈健, 翟玉龙. HY和USY分子筛对模拟油品中碱性氮化物的吸附行为[J].化工进展, 2014, 33(4):1035-1040. https://www.cnki.com.cn/qikan-HGJZ201404052.htmlXU Xiao-yu, SUN Yue, SHEN Jian, ZHAI Yu-long. Adsorption behavior of basic nitrides in model oil on HY and USY molecular sieves[J]. Chem Ind Eng Prog, 2014, 33(4):1035-1040. https://www.cnki.com.cn/qikan-HGJZ201404052.html [10] HONG X, TANG. Adsorptive denitrogenation of diesel oil using a modified NaY molecular sieve[J]. Petrol Sci Technol, 2015, 33:1471-1478. doi: 10.1080/10916466.2015.1076844 [11] 唐磊, 纪桂杰, 沈健. W-SBA-15的制备及其吸附脱氮性能研究[J].石油炼制与化工, 2015, 46(8):76-80. http://www.cnki.com.cn/Article/CJFDTotal-SYLH201508031.htmTANG Lei, JI Gui-jie, SHEN Jian. Study on W-SBA-15 preparation and its adsorptive denitrificationi performance[J]. Pet Process Petroche, 2015, 46(8):76-80. http://www.cnki.com.cn/Article/CJFDTotal-SYLH201508031.htm [12] 罗资琴, 郑晓明, 田放, 王辉. W-SBA-15分子筛吸附脱除焦化蜡油中碱性氮化物的实验研究[J].炼油技术与工程, 2017, 47(3):61-64. http://www.cnki.com.cn/Article/CJFDTotal-SXHG201507026.htmLUO Zi-qin, ZHENG Xiao-ming, TIAN Fang, WANG Hui. Experimental research on adsorption performances of the W-SBA-15 zeolite for basic nitrogen compounds in CGO[J]. Pet Refinery Eng, 2017, 47(3):61-64. http://www.cnki.com.cn/Article/CJFDTotal-SXHG201507026.htm [13] 王云芳, 步长娟, 迟志明, 李倩. Al-MCM-41介孔分子筛吸附喹啉的性能[J].化工学报, 2015, 66(9):3597-3604. http://d.old.wanfangdata.com.cn/Periodical/hgxb201509043WANG Yun-fang, BU Chang-juan, CHI Zhi-ming, LI Qian. Adsorption of quinoline on zeolite AL-MCM-41[J]. CIESC J, 2015, 66(9):3597-3604. http://d.old.wanfangdata.com.cn/Periodical/hgxb201509043 [14] 迟志明. 介孔分子筛用于柴油吸附脱氮的基础研究[D]. 北京: 中国石油大学, 2010. http://www.wanfangdata.com.cn/details/detail.do?_type=degree&id=Y1777883CHI Zhi-ming. Basic research of Al-MCM-41 molecular sieve for denitrogenation of diese oil[D]. Beijing: China University of Petroleum, 2010. http://www.wanfangdata.com.cn/details/detail.do?_type=degree&id=Y1777883 [15] 庞海全, 李艳芳, 韩冬云, 金阳, 乔海燕, 曹祖宾.烷基化法脱除模拟柴油中氮化物的研究[J].精细石油化工, 2017, 34(2):67-70. http://www.cnki.com.cn/Article/CJFDTOTAL-SXJG200904022.htmPANG Hai-quan, LI Yan-fang, HAN Dong-yun, JIN Yang, QIAN Hai-yan, CAO Zu-bin. Research on removing nitrogen compounds from model diesel oil by alkylayion method[J]. Spec Petrochem, 2017, 34(2):67-70. http://www.cnki.com.cn/Article/CJFDTOTAL-SXJG200904022.htm [16] SINA Rashidi S, Nikou M R K, Anvaripour B. Adsorptive desulfurization and denitrogenation of model fuel using HPW and NiO-HPW modified aluminosilicate mesostructures[J]. Microporous Mesoporous Mater, 2015, 211:134-141. doi: 10.1016/j.micromeso.2015.02.041 [17] 洪新, 唐克.杂原子介孔Co-MCM-41分子筛的制备及其吸附脱氮性能[J].燃料化学学报. 2015, 43(6):720-727. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18646.shtmlHONG Xin, TANG Ke. Preparation and adsorption denitrification of heteroatoms mesoporous molecular sieve Co-MCM-41[J]. J Fuel Chem Technol, 2015, 43(6):720-727. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18646.shtml [18] TANG K, HONG X. Preparation and characterization of Co-MCM-41 and its adsorption removing basic nitrogen compounds from FCC diesel oil[J]. Energy Fuels. 2016, 30(6):4619-4624. doi: 10.1021/acs.energyfuels.6b00427 [19] 洪新, 唐克, 丁世洪.杂原子介孔Co-MCM-41分子筛的制备及其柴油深度吸附脱氮性能[J].燃料化学学报, 2016, 44(1):99-105. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18767.shtmlHONG Xin, TANG Ke. DING Shi-hong. Preparation and deep adsorption denitrification from diesel oil of heteroatoms mesoporous molecular sieve Co-MCM-41[J]. J Fuel Chem Technol, 2016, 44(1):99-105. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18767.shtml [20] 李少凯, 阮本玺, 沈健, 赵明飞.硅胶吸附脱除模拟燃料中的碱性氮化物[J].石油炼制与化工, 2013, 44(6):22-25. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=sylzyhg201306005LI Shao-kai, RUAN Ben-xi, SHEN Jian, ZHAO Ming-fei. Study on nitrogen compounds adsorption performance of silica gel[J]. Pet Process Petrochem, 2013, 44(6):22-25. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=sylzyhg201306005 -





 下载:
下载: