Phenol etherification with methanol to anisole over supported Cs catalysts
-
摘要: 研究了不同酸碱中心、载体、前躯体和负载量对负载铯催化剂上苯酚与甲醇醚化制苯甲醚反应行为的影响。结果表明,碱性中心比酸性中心具有更高的苯甲醚选择性,碱性中心的阳离子影响催化剂的苯甲醚选择性。载体影响铯离子的电子结合能,从而影响催化剂的醚化活性;铯离子的电子结合能越低,催化剂醚化活性越低;载体影响催化剂强碱性位数量,从而影响苯甲醚选择性;强碱性位数量越多,副反应越容易发生,苯甲醚选择性越低。不同前躯体制备的Cs/SiO2由于表面相对铯原子数量不同而活性不同;Cs/SiO2的单层负载量为1.0 mmol/g,超过单层负载量后催化剂的平均活性显著下降。Abstract: The effect of acid and basic sites, support, cesium precursor and cesium loading on the performance of supported Cs catalysts in the etherification of phenol with methanol to anisole was investigated. The results illustrate that the cations of basic sites play an important role in the selective conversion of phenol to anisole; the basic sites give higher selectivity to anisole than the acid sites. The catalytic activity in phenol etherification decreases with the increase of the cesium ion binding energy, which is related to the support used. Moreover, the support also has an influence on the amount of strong basic sites, which is related to the selectivity to anisole; high amount of strong basic sites may promote the side reaction and decrease the selectivity to anisole. Cs/SiO2 catalysts prepared with various precursors are different in the surface Cs/Si atomic ratio, which may also influence the catalytic activity in phenol etherification; if the cesium loading exceeds the monolayer dispersion of cesium on SiO2, which is nearly 1.0 mmol/g, the average activity of Cs/SiO2 in phenol etherification decreases greatly.
-
Key words:
- supported Cs catalyst /
- activity /
- selectivity /
- phenol /
- anisol /
- etherification
-
表 1 不同二氧化硅负载催化剂的苯酚转化率和产物选择性
Table 1 Phenol conversion and product selectivity on different SiO2 supported catalysts
Catalyst Conversion
x/%Selectivity s/% anisol 2-MA① 3/4-MA① o-cresol m/p-cresol xylenol others H4Si (W3O10)4/SiO2 9.0 22.7 1.2 0.6 36.7 8.4 5.7 24.7 AlCl3/SiO2 71.9 33.9 6.9 2.9 20.0 4.2 16.2 15.9 K/SiO2 6.3 95.4 0.7 0.3 1.5 0.3 0.2 1.6 Cs/SiO2 36.0 98.7 0.2 ~0.0 0.3 0.1 ~0.0 0.7 Ba/SiO2 15.0 88.0 0.5 0.3 7.0 1.6 0.4 2.2 SiO2 1.4 63.6 4.0 2.0 13.7 3.8 5.1 7.8 ①: 2-methylanisol (2-MA), 3-methylanisol (3-MA), 4-methylanisol (4-MA); note: 400 ℃, LHSV=1.0 h-1, N2 flow of 20 mL/min, TOS=5 h, H, Al, Ba, Cs, Rb, K, Na loading of 0.5 mmol/g (CH3COOCs as the precusor) 表 2 不同载体负载铯催化剂的苯酚转化率和产物选择性
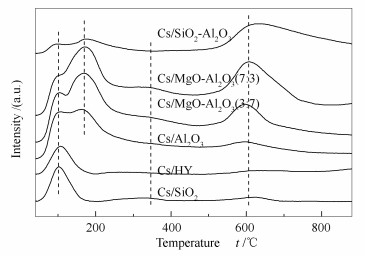
Table 2 Phenol conversion and product selectivity on the cesium catalysts with different supports
Support Conversion x/% Selectivity s/% anisol 2-MA① 3/4-MA① o-cresol m/p-cresol xylenol others Cs/HY 52.0 97.7 0.3 0.1 0.7 0.1 ~0.0 1.1 Cs/SiO2-Al2O3 52.6 67.8 4.6 1.9 16.8 2.6 3.8 2.5 Cs/SiO2 76.3 98.9 0.2 0.1 0.2 0.1 ~0.0 0.5 Cs/Al2O3 13.2 93.3 1.4 0.6 2.6 1.0 0.4 0.7 Cs/MgO-Al2O3(3:7) 12.4 90.1 0.4 0.2 6.1 2.0 0.2 1.0 Cs/MgO-Al2O3(7:3) 10.3 84.8 0.4 0.3 8.2 3.8 0.4 2.1 ①: 2-methylanisol (2-MA), 3-methylanisol (3-MA), 4-methylanisol (4-MA); note: 400 ℃, LHSV=1.0 h-1, N2 20 mL/min, TOS 5 h, Cs loading 1.0 mmol/g (CH3COOCs as the precusor) 表 3 不同载体负载的铯催化剂XPS表征
Table 3 XPS results of the cesium catalysts with different supports
Catalyst Cs 3d5/2/eV Cs/SiO2 725.0 Cs/Al2O3 724.6 Cs/ MgO-Al2O3(7:3) 724.5 表 4 不同前躯体催化剂的苯酚转化率和苯甲醚选择性
Table 4 Phenol conversion and anisol selectivity on the catalysts prepared with different precursors
Precusor Conversion
x/%Selectivity s/% anisol others CH3COOCs 36.0 98.7 1.3 CsNO3 50.7 99.0 1.0 Cs2CO3 53.1 98.8 1.2 note: 400 ℃, LHSV=1.0 h-1, N2 flow of 20 mL/min, TOS=5 h, Cs loading of 0.5 mmol/g 表 5 不同底物的转化率和产物选择性
Table 5 Conversion of different reactants and product selectivity
Reactant Conversion
x/%Selectivity s/% target product others Phenol 86.7 98.9 1.1 o-cresol 84.4 95.1 4.9 m-cresol 89.5 97.6 2.4 p-cresol 91.0 97.8 2.2 note: 400 ℃, LHSV 0.5 h-1, N2 flow of 20 mL/min, TOS=5 h, Cs loading of 1.0 mmol/g -
[1] 王汝成, 孙鸣, 刘巧霞, 马燕星, 冯光, 徐龙, 马晓迅.陕北中低温煤焦油中酚类化合物的提取与GC-MS分析[J].煤炭学报, 2011, 36(4): 664-669. http://www.cnki.com.cn/Article/CJFDTOTAL-MTXB201104032.htmWANG Ru-cheng, SUN Ming, LIU Qiao-xia, MA Yan-xing, FENG Guang, XU Long, MA Xiao-xun. Extraction and GC/MS analysis of phenolic compounds in low temperature coal tar from Northern Shaanxi[J]. J China Coal Soc, 2011, 36(4): 664-669. http://www.cnki.com.cn/Article/CJFDTOTAL-MTXB201104032.htm [2] 马宝岐, 任沛建, 杨占彪, 王树宽.煤焦油制燃料油品[M].北京:化学工业出版社, 2011.MA Bao-qi, REN Pei-jian, YANG Zhan-biao, WANG Shu-kuan. Preparation of Fuel Oil From Coal Tar[M]. Beijing: Chemical Industry Press, 2011. [3] 韩磊, 黄传峰, 杨天, 杨永佳, 李伟, 王孟艳, 焦友军, 任彩玲, 杨帆, 王永娟.一种煤焦油加工与煤液化联合装置:中国, 201520459906.X[P]. 2015-11-18.HAN Lei, HUANG Chuan-feng, YANG Tian, YANG Yong-jia, LI Wei, WANG Meng-yan, JIAO You-jun, REN Cai-ling, YANG Fan, WANG Yong-juan. A combined device for coal tar processing and coal liquefaction: CN, 201520459906.X[P]. 2015-11-18. [4] MORGAN J J, MEIGHAN M H. Extraction of phenols from tar oils by the caustic soda process[J]. Ind Eng Chem, 1925, 17: 696-700. doi: 10.1021/ie50187a018 [5] BERGERON P, HINMAN N. Technical and economic analysis of lignin conversion to methyl aryl ethers[J]. Appl Biochem Biotechnol, 1990, 24-25(1): 15-29. doi: 10.1007/BF02920230 [6] FARCASIU D, PRINCETON N J. Etherification catalyst: US, 4406821[P]. 1982-08-30. [7] DOLHYJ S R, PAPARIZOS C. Motor fuel additives derived from shale oil: US, 4407661[P]. 1983-10-4. [8] 王泽, 党丹, 宋文立, 林伟刚, 李松庚.一种催化剂及利用该催化剂对焦油进行提质的处理方法:中国, 201310342276.3[P]. 2013-08-07.WANG Ze, DANG Dan, SONG Wen-li, LIN Wei-gang, LI Song-geng. Catalyst and treatment method for upgrading tar by using catalyst: CN, 201310342276.3[P]. 2013-08-07. [9] 王利军, 唐祥海, 朱瑞芝, 潘履让.苯酚与甲醇合成苯甲醚沸石催化剂的研究[J].石油学报:石油加工, 1998, 14: 45-49. http://www.cnki.com.cn/Article/CJFDTOTAL-SXJG802.008.htmWANG Li-jun, TANG Xiang-hai, ZHU Rui-zhi, PAN Lü-rang. Research on zeolite catalyst for synthesis of methyl phenyl ether with phenol and methanol[J]. Acta Pet Sin (Pet Process Sect), 1998, 14: 45-49. http://www.cnki.com.cn/Article/CJFDTOTAL-SXJG802.008.htm [10] 邹秀晶, 朱小梅, 李雪梅, 王振旅, 刘钢, 贾明君, 张文祥.二氧化硅负载偏钨酸铵催化剂上邻苯二酚和甲醇气相单醚化反应[J].催化学报, 2008, 29(7): 671-676. http://www.cnki.com.cn/Article/CJFDTOTAL-CHUA200807017.htmZHOU Xiu-jing, ZHU Xiao-mei, LI Xue-mei, WANG Zhen-lü, LIU Gang, JIA Ming-jun, ZHANG Wen-xiang. Vapour-phase O-methylation of catechol with methanol on SiO2-supported ammonium metatungstate catalysts[J]. Chin J Catal, 2008, 29(7): 671-676. http://www.cnki.com.cn/Article/CJFDTOTAL-CHUA200807017.htm [11] SAMOLADA M C, GRIGORIADOU E, KIPARISSIDES Z, VASALOS I A. Selective O-alkylation of phenol with methanol over sulfates supported onγ-Al2O3[J]. J Catal, 1995, 152(1): 52-62. doi: 10.1006/jcat.1995.1059 [12] VELU S, SWAMY C S. Alkylation of phenol with methanol over magnesium-aluminium calcined hydrotalcites[J]. Appl Catal A: Gen, 1994, 119(2): 241-252. doi: 10.1016/0926-860X(94)85194-8 [13] SARALA DEVI G, GIRIDHAR D, REDDY B M. Vapour phase O-alkylation of phenol over alkali promoted rare earth metal phosphates[J]. J Mol Catal A: Chem, 2002, 181(1/2): 173-8. https://www.researchgate.net/publication/257868604_Alkali_promoted_rare_earth_metal_phosphates_for_vapour_phase_O-alkylation_of_-_and_-naphthols_with_methanol [14] 王艳力, 周周, 吴淑杰, 吴桐舜, 贾明君, 张文祥. SiO2负载K催化剂上苯酚和甲醇的醚化反应[J].石油化工, 2004, 33: 170-171.WANG Yan-Li, ZHOU Zhou, WU Shu-jie. Phenol etherification with methanol on SiO2 supported K catalyst[J]. Petrkchem Technol, 2004, 33: 170-171. [15] BAL R, SIVASANKER S. Vapour phase selective O-alkylation of phenol over alkali loaded silica[J]. Appl Catal A: Gen, 2003, 246(2): 373-82. doi: 10.1016/S0926-860X(03)00082-6 [16] AZZOUZ A, NISTOR D, MIRON D, URSU A V, SAJIN T, MONETTE F, NIQUETTE P, HAUSLER R. Assessment of acid-base strength distribution of ion-exchanged montmorillonites through NH3 and CO2-TPD measurements[J]. Thermochim Acta, 2006, 449(1/2): 27-34. https://www.researchgate.net/publication/229145432_Assessment_of_acid-base_strength_distribution_of_ion-exchanged_montmorillonites_through_NH3_and_CO2-TPD_measurements [17] 辛勤, 罗孟飞.现代催化研究方法[M].北京:科学出版社, 2009.XIN Qin, LUO Meng-fei. Modern Catalytic Research Methods[M]. Beijing: Science Press, 2009. [18] 吴娟霞, 徐华, 张锦.拉曼光谱在石墨系结构表征中的应用[J].化学学报, 2014, 72: 301-318. doi: 10.6023/A13090936WU Juan-xia, XU Hua, ZHANG Jin. Raman spectroscopy of graphene[J]. Acta Chim Sin, 2014, 72: 301-318. doi: 10.6023/A13090936 [19] DAVIS Z D, TATARCHUK B J. Understanding the dispersion of Ag on high surface area TiO2 supports using XPS intensity ratios[J]. Appl Surf Sci, 2015, 353: 679-85. doi: 10.1016/j.apsusc.2015.06.086 [20] 刘英骏, 赵明, 郭沁林, 桂琳琳, 谢有畅, 唐有祺. WO3在γ-Al2O3表面的分散状态和最大分散量[J].化学学报, 1985, 43: 728-732.LIU Ying-jun, ZHAO Ming, GUO Qin-lin, GUI Lin-lin, XIE You-chang, TANG You-qi. Determination of the maximum monolayer dispersion and dispersed state for WO3 onγ-Al2O3[J]. Acta Chim Sin, 1985, 43: 728-732. -





 下载:
下载: