In-situ reaction between arsenic/selenium and minerals in fly ash at high temperature during blended coal combustion
-
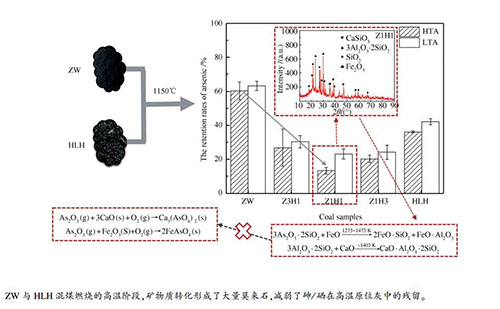
摘要: 为了研究混煤燃烧过程中痕量元素与飞灰中矿物质的原位反应,选取烟煤(HLH)、无烟煤(ZW)及其混煤在1150 ℃时的沉降炉中进行燃烧,并分别收集和分析了高温段灰分(HTA)和低温段灰分(LTA)中砷和硒残留率。结果表明,砷在高温段灰分中的残留率低于低温段灰,说明在烟气冷却过程中砷会被灰重新吸附。ZW、Z3H1、Z1H1、Z1H3、HLH的高温段灰中砷的残留率分别为60.31%、26.85%、13.29%、20.23%、36.11%,说明混煤的高温段灰比原煤更难捕获砷。同时,硒在五种煤样的高温段灰中的残留率分别为24.68%、23.60%、20.58%、15.19%和38.13%,其残留规律与砷相同。此外,X射线衍射(XRD)分析结果表明,混煤燃烧过程中矿物形态发生了明显变化。与原煤不同的是,混煤的HTA中出现了莫来石,且莫来石的峰值随着混煤中ZW比例的增加而增强。这与HTA中砷和硒的残留趋势一致。说明在混煤燃烧过程中,矿物质种类的变化以及矿物质与痕量元素的原位反应对砷和硒的排放有显著影响。Abstract: Blended coal combustion technology was extensively used in coal-fired power plants in China. In order to investigate the in-situ reaction between trace elements and minerals in fly ash during blended coal combustion, a bituminous (HLH), anthracite (ZW) and the blended coal of these two parent coals were combusted in a drop tube furnace at 1150 ℃. The ash gathered at high temperature segment (HTA) and low temperature segment (LTA) of the furnace were analyzed, respectively. The results indicated that the retention rates of arsenic in HTA were lower than that in LTA, which suggested that arsenic would be re-absorbed by ash during cooling down of flue gas. For HTA the retention rates of arsenic in ash of ZW, Z3H1, Z1H1, Z1H3, HLH were 60.31%, 26.85%, 13.29%, 20.23% and 36.11%, respectively. The arsenic was more difficult to be captured by HTA of blended coal than that of parent coal. As for selenium, the retention rates in HTA of five coal samples were 24.68%, 23.60%, 20.58%, 15.19% and 38.13%, which had the same retention law as arsenic. The results of X-ray diffraction (XRD) demonstrated that the mineral morphology was changed obviously during blended coal combustion. Unlike parent coal, mullite appeared in HTA of blended coal, and peak of mullite was enhanced with proportion of ZW increased in blended coal. It was consistent with the trend of retention of As and Se in HTA. It illustrated that change of mineral species and in-situ reaction between minerals and trace elements significantly affected emission of arsenic and selenium during blended coal combustion.
-
Key words:
- blended coal /
- arsenic /
- selenium /
- mineral /
- in-situ reaction
-
Table 1 Ultimate/proximate analysis of the samples
Sample Ultimate analysis /% Proximate analysis wad /% Content /(μg·g-1) C H N S O* M A V FC As Se ZW 34.13 1.47 0.40 - 17.85 2.63 43.52 8.79 45.06 1.98 0.68 HLH 49.96 5.40 1.07 0.62 4.87 4.81 32.84 36.80 25.77 18.07 0.20 *: by different, ad: air dry, -: not detected Table 2 Ash composition of the samples
Sample Composition w/% SiO2 Al2O3 CaO Fe2O3 Na2O K2O MgO SO3 TiO2 ZW 42.64 21.03 6.92 9.03 5.60 2.85 1.15 3.99 1.34 HLH 56.20 14.98 6.45 12.80 1.52 2.01 0.71 6.36 0.95 Table 3 Sequential chemical extraction method
Step Solid phases Extraction(0.5 g of coal) Demonstration S1 exchangeable 15 mL of 1 mol/L MgCl2 oscillated 4 h at room temperature, then centrifuged
10 min at 3000 r/minS2 sulfide-bound 15 mL of 12.5% HNO3 (W/W) 0.5 h at 95 ℃ with intermittent oscillation, then centrifuged
10 min at 3000 r/minS3 organic-bound 10 mL of HNO3 (pH=2) and
5 mL of 30% H2O25 h at 80-85 ℃ with intermittent oscillation, then centrifuged
10 min at 3000 r/minS4 residual 8 mL of HNO3 and 2 mL of HF digested in the microwave digestion system -
[1] XUAN W W, WANG H N, XIA D H. Depolymerization mechanism of CaO on network structure of synthetic coal slags[J]. Fuel Process Technol, 2019, 187: 21-27. http://www.sciencedirect.com/science/article/pii/S0378382018316230 [2] WANG S B, LUO K L, WANG X, SUN Y Z. Estimate of sulfur, arsenic, mercury, fluorine emissions due to spontaneous combustion of coal gangue: An important part of Chinese emission inventories[J]. Environ Pollut, 2016, 209: 107-113. https://www.sciencedirect.com/science/article/pii/S026974911530186X [3] ZHAO S L, DUAN Y F, CHEN L, LI Y N, YAO T, LIU S, LIU M, LU J H. Study on emission of hazardous trace elements in a 350 MW coal-fired power plant. Part 2. arsenic, chromium, barium, manganese, lead[J]. Environ Pollut, 2017, 226: 404-411. https://www.sciencedirect.com/science/article/pii/S0269749117303354 [4] HAN J, ZHANG L, ZHAO B, QIN L B, WANG Y, XING F T. The N-doped activated carbon derived from sugarcane bagasse for CO2 adsorption[J]. Ind Crops Prod, 2019, 128: 290-297. https://www.sciencedirect.com/science/article/pii/S0926669018310033 [5] LEELARUNGROJ K, LIKITLERSUANG S, CHOMPOORAT T, JANJAROEN D. Leaching mechanisms of heavy metals from fly ash stabilised soils[J]. Waste Manage Res, 2018, 36(7): 616-623. doi: 10.1177/0734242X18775494 [6] EVANDRO D S, LI S W, LETUZIA D O, JULIA G, DONG X L, ANN C W, TIMOTHY G T, LENA Q M. Metal leachability from coal combustion residuals under different pHs and liquid/solid ratios[J]. J Hazard Mater, 2018, 341: 66-74. https://www.sciencedirect.com/science/article/pii/S0304389417305095 [7] GB/T13233-2011, Emission standard of air pollutants for thermal power plants[S]. [8] GB3095-2012, Ambient air quality standards[S]. [9] TANG Q, LIU G J, YAN Z C, RUOYU S. Distribution and fate of environmentally sensitive elements (arsenic, mercury, stibium and selenium) in coal-fired power plants at Huainan, Anhui, China[J]. Fuel, 2012, 95: 334-339. http://www.sciencedirect.com/science/article/pii/S0016236111008210 [10] CONTRERAS M L, AROSTEGUI J M, ARMESTO L. Arsenic interactions during co-combustion processes based on thermodynamic equilibrium calculations[J]. Fuel, 2009, 88(3): 539-546. http://www.sciencedirect.com/science/article/pii/S0016236108003918 [11] GERALD P H, FRANK E H, NARESH S, ZHAO J M. Speciation of arsenic and chromium in coal and combustion ash by XAFS spectroscopy[J]. Fuel Process Technol, 1994, 39(1): 47-62. https://www.sciencedirect.com/science/article/pii/0378382094901716 [12] ROBERT A Z, ANDREA L F, GREGORY P M, ISABELLE K B. Mode of occurrence of arsenic in feed coal and its derivative fly ash, Black Warrior Basin, Alabama[J]. Fuel, 2007, 86(4): 560-572. http://www.sciencedirect.com/science/article/pii/S0016236106003115 [13] YAN R, DANIEL G, GILLES F, WANG Y M. Behavior of selenium in the combustion of coal or coke spiked with Se[J]. Combust Flame, 2004, 138(1): 20-29. http://www.sciencedirect.com/science/article/pii/S0010218004000793 [14] CONSTANCE S, BRYDGER V O, JOST O L W, ADEL S. Modeling the behavior of selenium in pulverized-coal combustion systems[J]. Combust. Flame, 2010, 157(11): 2095-2105. [15] ZHOU C C, LIU G J, XU Z Y, SUN H, PAUL K S L. Retention mechanisms of ash compositions on toxic elements (Sb, Se and Pb) during fluidized bed combustion[J]. Fuel, 2018, 213: 98-105. http://www.sciencedirect.com/science/article/pii/S0016236117313674 [16] ANNA A R, OLEG K, EVGUENⅡ I K, DAVID T P, WAYNE S. In situ evaluation of inorganic matrix effects on the partitioning of three trace elements (As, Sb, Se) at the outset of coal combustion[J]. Energy Fuels, 2011, 25(9/10): 4290-4298. https://www.sciencedirect.com/science/article/pii/S0016236118320271 [17] KUO J H, LIN C L, WEY M Y. Effect of particle agglomeration on heavy metals adsorption by Al- and Ca-based sorbents during fluidized bed incineration[J]. Fuel Process Technol, 2011, 92(10): 2089-2098. http://www.sciencedirect.com/science/article/pii/S0378382011002347 [18] IKEDA M, MAKINO H, MORINAGA H, HIGASHIYAMA K, KOZAI Y. Emission characteristics of NOx and unburned carbon in fly ash during combustion of blends of bituminous/sub-bituminous coals[J]. Fuel, 2003, 82(15): 1851-1857. https://www.sciencedirect.com/science/article/pii/S0016236103001704 [19] KUROSE R, IKEDA M, MAKINO H. Combustion characteristics of high ash coal in a pulverized coal combustion[J]. Fuel, 2001, 80(10): 1447-1455. https://www.sciencedirect.com/science/article/pii/S0016236101000205 [20] ZHU C, TU H, BAI Y, MA D, ZHAO Y G. Evaluation of slagging and fouling characteristics during Zhundong coal co-firing with a Si/Al dominated low rank coal[J]. Fuel, 2019, 254: 115730. http://www.sciencedirect.com/science/article/pii/S0016236119310828 [21] DUAN L B, SUN H C, JIANG Y, EDWARD A, ZHAO C S. Partitioning of trace elements, As, Ba, Cd, Cr, Cu, Mn and Pb, in a 2.5 MWth pilot-scale circulating fluidised bed combustor burning an anthracite and a bituminous coal[J]. Fuel Process Technol, 2016, 146: 1-8. http://www.sciencedirect.com/science/article/pii/S0378382016300467 [22] HAN J K, YU D X, WU J Q, YU X, LIU F Q, WANG J H, XU M H. Fine ash formation and slagging eeposition during combustion of Silicon-rich biomasses and their blends with a low-rank coal[J]. Energy Fuels, 2019, 33(7): 5875-5882. doi: 10.1021/acs.energyfuels.8b04193 [23] GB3058-2008, Determination of arsenic in coal[S]. [24] GB/T16415-2008, Determination of selenium in coal-Hydride generation-atomic absorption method[S]. [25] ZOU C, WANG C B, LIU H M, WANG H F, ZHANG Y. Effect of volatile and ash contents in coal on the volatilization of arsenic during isothermal coal combustion[J]. Energy Fuels, 2017, 31(11): 12831-12838. doi: 10.1021/acs.energyfuels.7b02187 [26] LIU H M, WANG C B, ZHANG Y, HUANG X Z, GUO Y C, WANG J W. Experimental and modeling study on the volatilization of arsenic during co-combustion of high arsenic lignite blends[J]. Appl Therm Eng, 2016, 108: 1336-1343. https://www.sciencedirect.com/science/article/pii/S135943111631331X [27] LIU H M, WANG C B, ZOU C, ZHANG Y, WANG J W. Simultaneous volatilization characteristics of arsenic and sulfur during isothermal coal combustion[J]. Fuel, 2017, 203: 152-161. https://www.sciencedirect.com/science/article/pii/S0016236117305276 [28] DÍAZ-SOMOANO M, LÓPEZ-ANTÓN M A, HUGGINS F, MARTÍNEZ-TARAZONA M R. The stability of arsenic and selenium compounds that were retained in limestone in a coal gasification atmosphere[J]. J Hazard Mater, 2010, 173(1): 450-454. https://www.sciencedirect.com/science/article/abs/pii/S0304389409014034 [29] ROSALES C, BARRERA-DÍAZ C E, BILYEU B, VARELA-GUERRERO V. A review on Cr(Ⅵ) adsorption using inorganic materials[J]. Am J Anal Chem, 2013, 4(7): 8-16. https://file.scirp.org/pdf/AJAC_2013070217041420.pdf [30] ANN G K, GEORGE K. The silicate/non-silicate distribution of metals in fly ash and its effect on solubility[J]. Fuel, 2004, 83(17): 2285-2292. https://www.sciencedirect.com/science/article/pii/S0016236104001590 [31] YANG Y H, HU H Y, XIE K, HUANG Y D, LIU H, LI X, YAO H, NARUSE I. Insight of arsenic transformation behavior during high-arsenic coal combustion[J]. Proc Combust Inst, 2019, 37(4): 4443-4450. https://www.sciencedirect.com/science/article/pii/S1540748918304826 [32] TIAN C, GUPTA R, ZHAO Y C, ZHANG J Y. Release behaviors of arsenic in fine particles generated from a typical high-arsenic coal at a high temperature[J]. Energy Fuels, 2016, 30(8): 6201-6209. doi: 10.1021/acs.energyfuels.6b00279 [33] SEAMES W, WENDT J O L. Regimes of association of arsenic and selenium during pulverized coal combustion[J]. Proc Combust Inst, 2007, 31(2): 2839-2846. https://www.sciencedirect.com/science/article/pii/S1540748906003294 [34] SENIOR C L, BOOL L E, SRINIVASACHAR S, PEASE B R, PORLE K. Pilot scale study of trace element vaporization and condensation during combustion of a pulverized sub-bituminous coal[J]. Fuel Process Technol, 2000, 63(2): 149-165. https://www.sciencedirect.com/science/article/pii/S0378382099000946 [35] ISKHAKOV K A, SCHASTLIVTSEV E L, KONDRATENKO Y A. Classification of the mineral components of coal[J]. Coke Chem, 2009, 51(12): 485-487. http://www.ingentaconnect.com/content/ssam/1068364x/2008/00000051/00000012/art00004 [36] ZHAN Z H, LIU X W, YAO H. Excluded mineral matter transformation mechanism and kinetics during coal combustion[J]. J Combust Sci Technol, 2007, 13(4): 355-359. https://www.sciencedirect.com/science/article/pii/S0016236102000273 [37] ZHANG R, LEI K, YE B Q, CAO J, LIU D. Combustion characteristics and synergy behaviors of biomass and coal blending in oxy-fuel conditions: A single particle co-combustion method[J]. Sci China: Technol Sci, 2018, 61(11): 1723-1731. doi: 10.1007/s11431-018-9214-9 [38] SENIOR C L, FLAGAN R C. Ash vaporization and condensation during combustion of a suspended coal particle[J]. Aerosol Sci Technol, 2007, 1(4): 371-383. doi: 10.1080/02786828208958602 [39] HELBLE J, NEVILLE M, SAROFIM A F. Aggregate formation from vaporized ash during pulverized coal combustion[J]. Symp Combust, 1988, 21(1): 411-417. http://www.sciencedirect.com/science/article/pii/S0082078488802686 [40] LI Y W, ZHAO C S, XIN W, LU D F. Theoretical and experimental study of aggregation and removal of fuel coal PM10 in magnetic fields[J]. J Eng Therm Energy Power, 2007, 22(2): 176-180. [41] SONG B, SONG M, CHEN D D, CAO Y, MENG F Y, WEI Y X. Retention of arsenic in coal combustion flue gas at high temperature in the presence of CaO[J]. Fuel, 2020, 259: 116249. http://www.sciencedirect.com/science/article/pii/S0016236119316035 [42] WU X J, ZHANG Z X, CHEN Y S, ZHOU T, FAN J J, PIAO G L, KOBAYASHI N, MORI S, ITAYA Y. Main mineral melting behavior and mineral reaction mechanism at molecular level of blended coal ash under gasification condition[J]. Fuel Process Technol, 2010, 91(11): 1591-1600. http://www.sciencedirect.com/science/article/pii/S0378382010002110 [43] SHAH P, STREZOV V, STEVANOV C, NELSON P F. Speciation of arsenic and selenium in coal combustion products[J]. Energy Fuels, 2007, 21(2): 506-512. doi: 10.1021/ef0604083 [44] CONTRERAS M L, GARCÍA-FRUTOS F J, BAHILLO A. Oxy-fuel combustion effects on trace metals behaviour by equilibrium calculations[J]. Fuel, 2013, 108: 474-483. http://www.researchgate.net/publication/273446035_OXY-FUEL_COMBUSTION_EFFECTS_ON_TRACE_METALS_BEHAVIOUR_BY_EQUILIBRIUM_CALCULATIONS [45] HAN J, XIONG Z J, ZHAO B, LIANG Y S, WANG Y, QIN L B. A prediction of arsenic and selenium emission during the process of bituminous and lignite coal co-combustion[J/OL]. Chem Pap, 2020. DOI: 10.1007/s11696-020-01058-9. [46] SENIOR C L, BOOL L E, MORENCY J R. Laboratory study of trace element vaporization from combustion of pulverized coal[J]. Fuel Process Technol, 2000, 63(2): 109-124. http://www.sciencedirect.com/science/article/pii/S0378382099000922 [47] ITSKOS G, KOUKOUZAS N, VASILATOS C, MEGREMI I, MOUTSATSOU A. Comparative uptake study of toxic elements from aqueous media by the different particle-size-fractions of fly ash[J]. J Hazard Mater, 2010, 183(1): 787-792. http://www.ncbi.nlm.nih.gov/pubmed/20724071 [48] FURUZONO T, NAKAJIMA T, FUJISHIMA H, TAKANASHI H, OHKI A. Behavior of selenium in the flue gas of pulverized coal combustion system: Influence of kind of coal and combustion conditions[J]. Fuel Process Technol, 2017, 167: 388-394. http://www.sciencedirect.com/science/article/pii/S0378382017305532 [49] FAN Y M, ZHUO Y Q, LI L L. SeO2 adsorption on CaO surface: DFT and experimental study on the adsorption of multiple SeO2 molecules[J]. Appl Surf Sci, 2017, 420: 465-471. http://adsabs.harvard.edu/abs/2017ApSS..420..465F [50] QUEROL X, FERNANDEZ-TURIEL J L, LÓPEZ-SOLER A. Trace elements in coal and their behaviour during combustion in a large power station[J]. Fuel, 1995, 74(3): 331-343. http://www.sciencedirect.com/science/article/pii/001623619593464O [51] LI Y Z, TONG H L, ZHUO Y Q, CHEN C H, XU X C. Simultaneous removal of SO2 and trace SeO2 from flue gas: Effect of product layer on mass transfer[J]. Environ Sci Technol, 2006, 40(13): 4306-4311. http://www.tandfonline.com/servlet/linkout?suffix=CIT0095&dbid=8&doi=10.1080%2F00206814.2017.1362671&key=16856751 -





 下载:
下载: